Preparation method and purification method of azacyclo-sulfide
A technology of nitrogen heterocyclic compounds and nitrogen heterocycles, applied in chemical instruments and methods, chemical/physical/physical chemical mobile reactors, chemical/physical processes, etc., can solve harsh conditions, low reaction efficiency, complex operations, etc. problems, to achieve the effect of ensuring the circulation function, expanding the scope, and preventing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
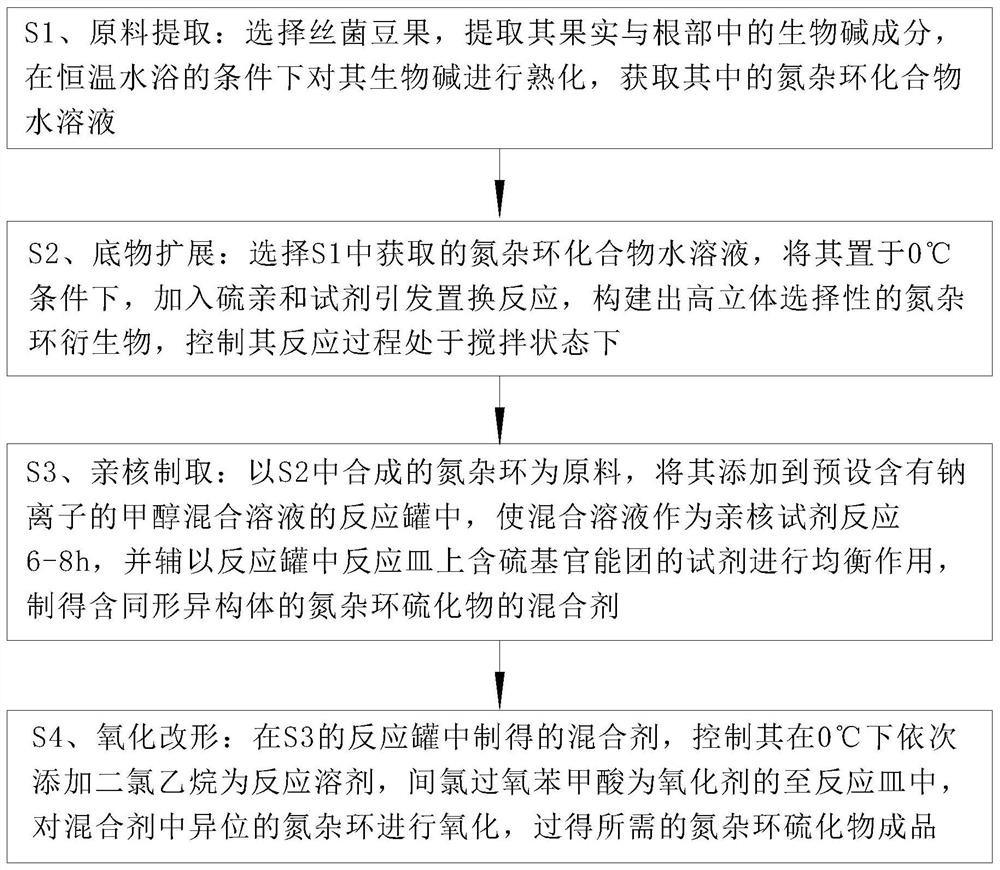
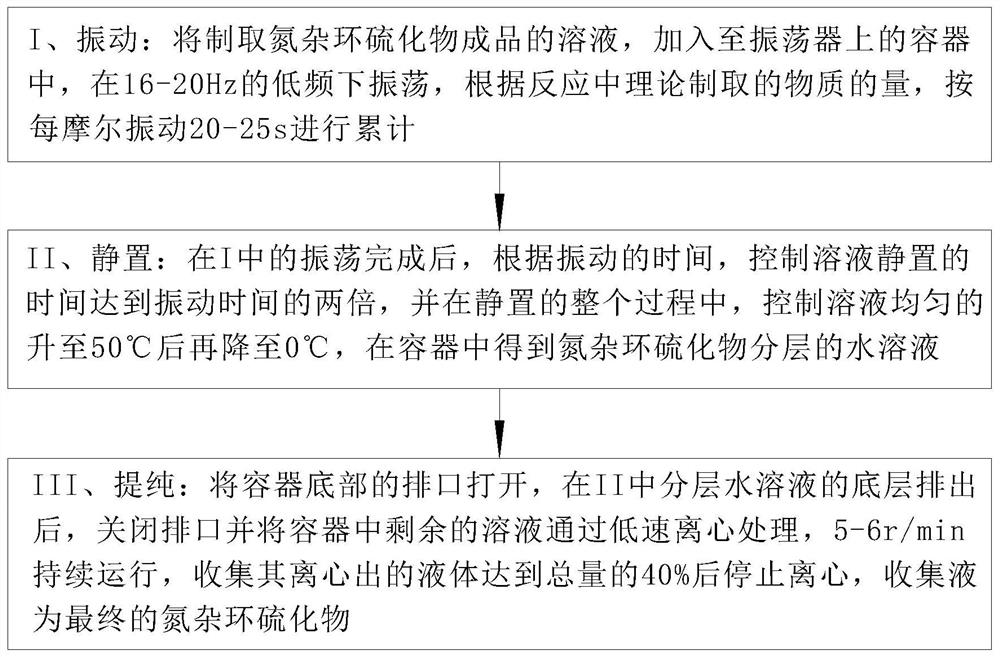
[0032] Such as Figure 1 to Figure 6 Shown, the preparation method of a kind of azacyclic sulfide of the present invention, the method step is as follows:
[0033] S1. Extraction of raw materials: select the mycelium bean fruit, extract the alkaloid components in the fruits and roots, and ripen the alkaloids in a constant temperature water bath to obtain the nitrogen heterocyclic compound aqueous solution; extract from natural plants Alkaloids are beneficial to obtain a large amount of nitrogen heterocyclic compounds, and the aging treatment in a water bath helps to improve the chemical activity of nitrogen heterocyclic compounds, which is convenient for the subsequent reaction process;
[0034] S2. Substrate expansion: select the nitrogen heterocyclic compound aqueous solution obtained in S1, place it at 0°C, add a sulfur affinity reagent to initiate a displacement reaction, construct a nitrogen heterocyclic derivative with high stereoselectivity, and control its The reactio...
Embodiment approach
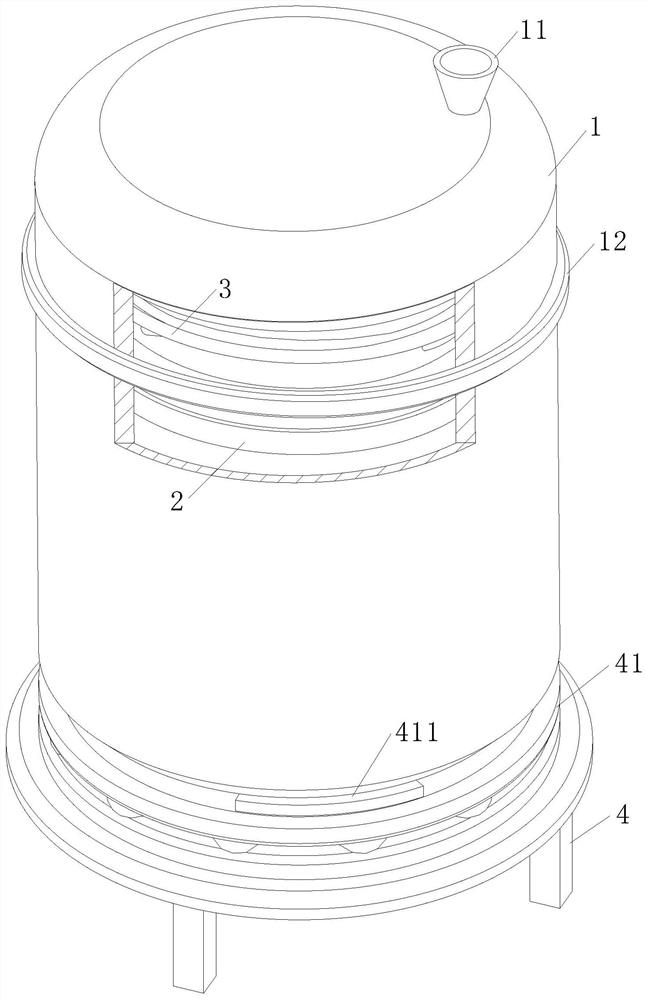
[0038] As an embodiment of the present invention, a plate blocking cover 311 is provided in the titration plate 31, and the plate blocking cover 311 closes the titration port in the titration plate 31; a convex ring is provided on the circumference of the plate blocking cover 311 312, the outer wall of the tank body 1 corresponding to the protruding ring 312 is provided with a fitted magnetic ring 12, and the magnetic ring 12 drives the blocking plate cover 311 to rise in the titration pan 31 through the protruding ring 312; on the outer surface of the protruding ring 312 A rubber pad 313 is provided, and the rubber pad 313 blocks the nozzle between the shunt tube 32 and the titration pan 31 when the blocking cover 311 rises; in the titration pan 31 corresponding to the reaction vessel 2 in the tank body 1, it is necessary to control the multi-layer The titration plate 31 and the reaction vessel 2 will circulate the reaction of the substrate solution; maintain the production of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com