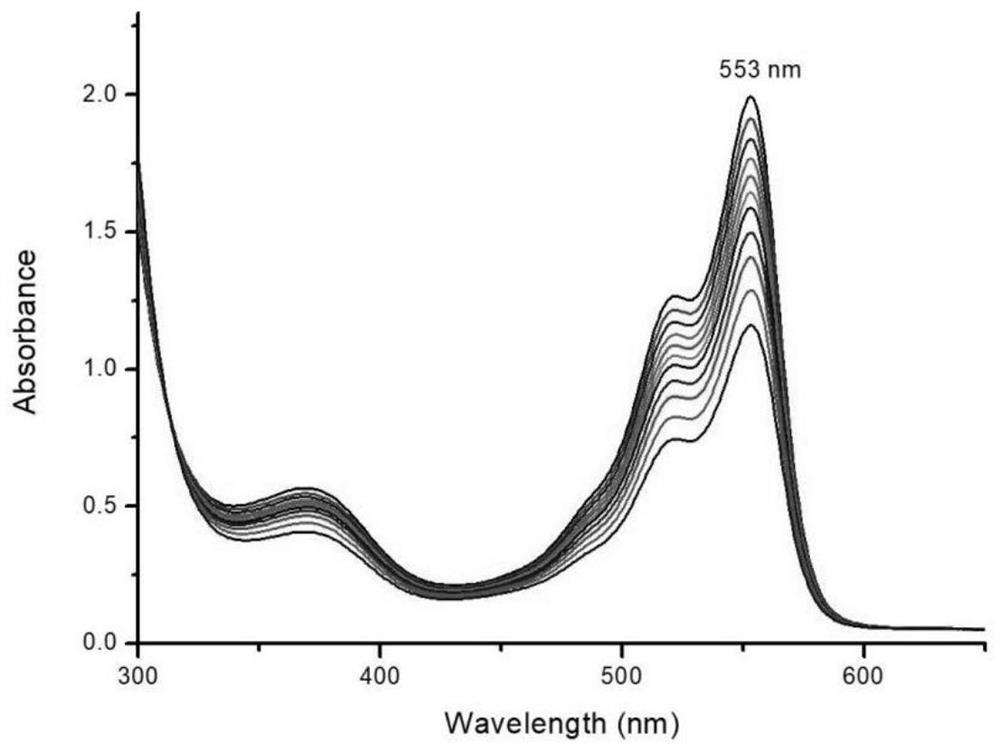
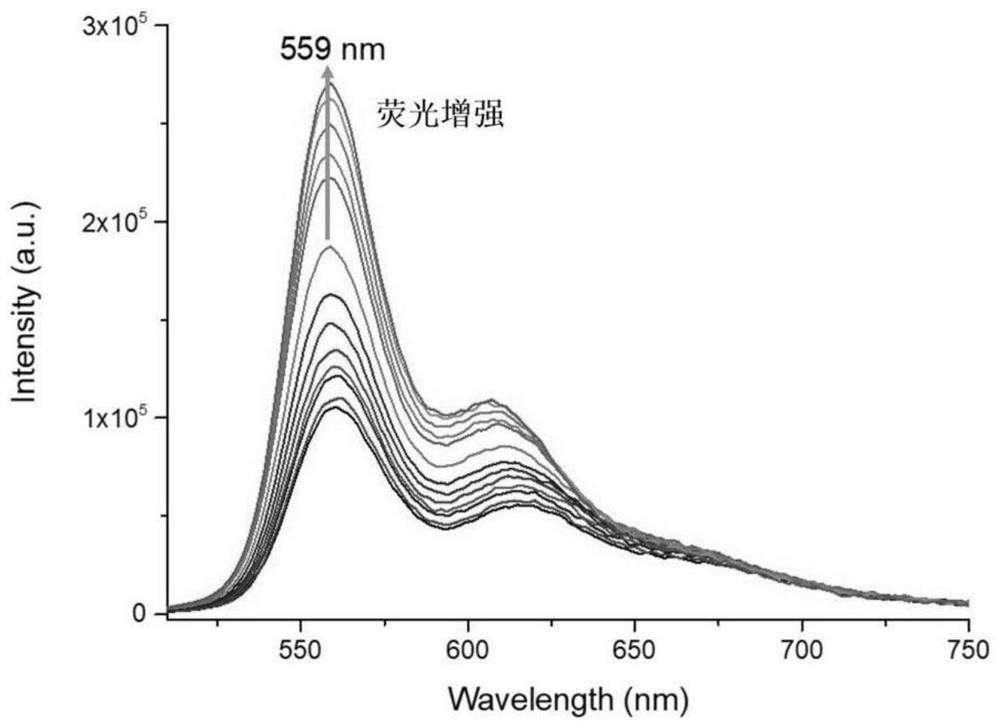
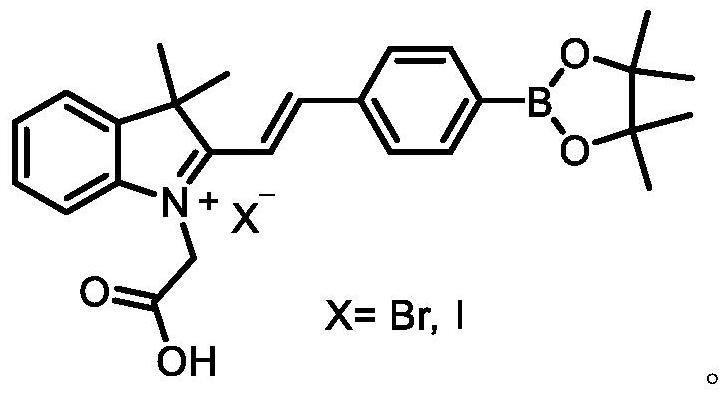
Indole hydrogen peroxide fluorescent probe and preparation method thereof
A technology of indole hydrogen peroxide and fluorescent probes, applied in the field of biological fluorescent probes, can solve the problems of poor stability, low quantum yield, complex synthesis process, etc., achieve low cost, simple synthesis method, fluorescent quantum production high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Weigh 1 g of 2,3,3-trimethylindole into a 100 ml flask, and add 20 ml of dry acetonitrile. After dissolving, 1.17 g of iodoacetic acid was added, and the mixture was reacted at 60° C. under reflux for 4 hours. After the reaction was completed, most of the acetonitrile was removed under vacuum distillation, and the product was dropped into anhydrous ether, and a black solid was precipitated. After suction filtration, the filter cake was washed three times with anhydrous ether to obtain the pure product iodo-1-carboxymethyl-2,3,3-trimethylindole with a yield of 85%.
[0034] (2) Weigh 1.5 g of 4-formylphenylboronic acid and 1.18 g of pinacol in a 500 ml flask, and add 150 ml of toluene. The mixture was reacted at 80°C under reflux for 8 hours. After the reaction was completed, the solvent toluene was removed under reduced pressure distillation, and white 4-formylphenylboronic acid pinacol ester was precipitated, which was directly used for the next reaction.
[003...
Embodiment 2
[0038] (1) Weigh 1 g of 2,3,3-trimethylindole into a 100 ml flask, and add 20 ml of dry acetonitrile. After dissolving, 0.86 g of bromoacetic acid was added, and the mixture was reacted under reflux at 100° C. for 4 hours. After the reaction was completed, most of the acetonitrile was removed under vacuum distillation, and the product was dropped into anhydrous ether, and a black solid was precipitated. After suction filtration, the filter cake was washed three times with anhydrous ether to obtain the pure bromo-1-carboxymethyl-2,3,3-trimethylindole with a yield of 88%.
[0039](2) Weigh 1.5 g of 4-formylphenylboronic acid and 1.18 g of pinacol in a 500 ml flask, and add 150 ml of toluene. The mixture was reacted at 120° C. under reflux for 8 hours. After the reaction was completed, the solvent toluene was removed under reduced pressure distillation, and white 4-formylphenylboronic acid pinacol ester was precipitated, which was directly used for the next reaction.
[0040] ...
Embodiment 3
[0043] (1) Weigh 1 g of 2,3,3-trimethylindole into a 100 ml flask, and add 20 ml of dry acetonitrile. After being dissolved, 1.17 g of iodoacetic acid was added, and the mixture was reacted at 60° C. under reflux for 12 hours. After the reaction was completed, most of the acetonitrile was removed under vacuum distillation, and the product was dropped into anhydrous ether, and a black solid was precipitated. After suction filtration, the filter cake was washed three times with anhydrous ether to obtain the pure product iodo-1-carboxymethyl-2,3,3-trimethylindole with a yield of 85%.
[0044] (2) Weigh 1.5 g of 4-formylphenylboronic acid and 1.18 g of pinacol in a 500 ml flask, and add 150 ml of toluene. The mixture was reacted at 120° C. under reflux for 16 hours. After the reaction was completed, the solvent toluene was removed under reduced pressure distillation, and white 4-formylphenylboronic acid pinacol ester was precipitated, which was directly used for the next reactio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com