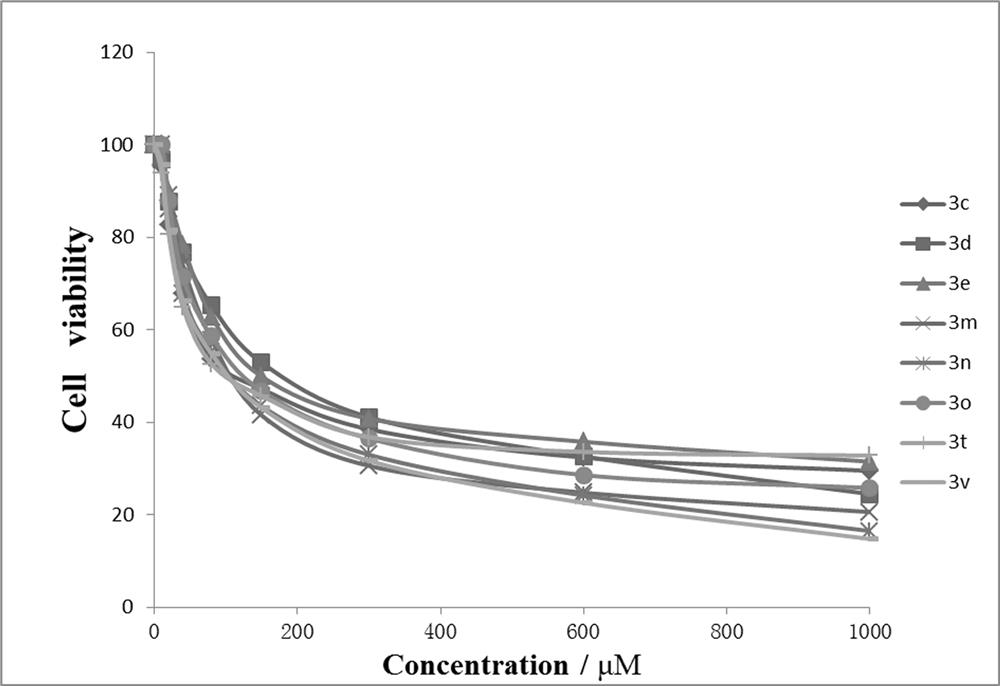
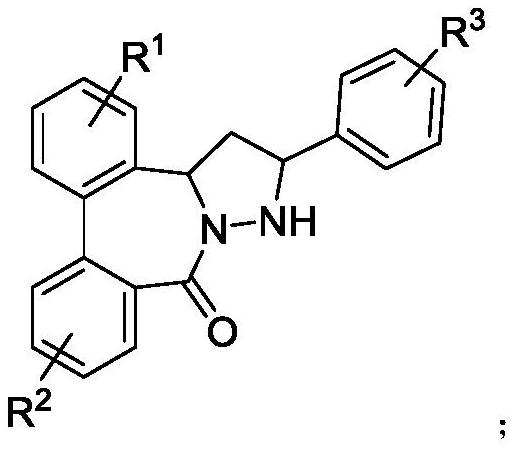
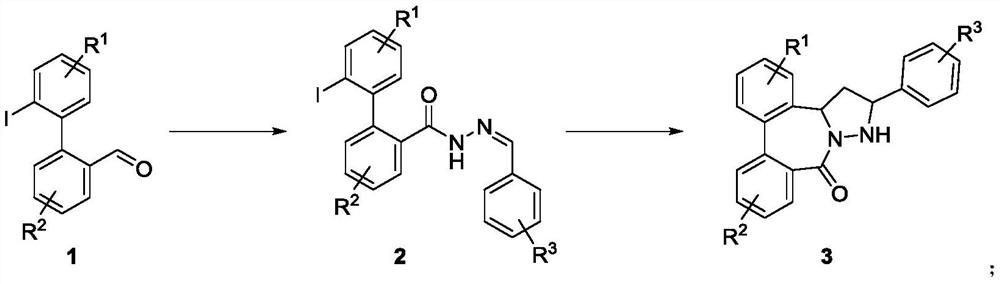
Dibenzocaprolactam pyrazolidine compound, preparation method and application thereof
A technology of pyrazolidine and caprolactam, which is applied in the field of pesticides, can solve the problems that the molting process cannot be carried out completely, kill pests and the like, and achieve the effects of good application prospect, good insecticidal activity and good biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of compound 3a:
[0035] Weigh 5mmol of 2'-iodo-[1,1'-biphenyl]-2-carbaldehyde into a reaction flask, add 30mL of ethanol, then add 6mmol of benzohydrazide, stir the reaction at 80°C overnight, and detect by TLC The reaction is complete, the reaction is cooled to room temperature, the precipitated solid is filtered, washed with petroleum ether, and the acylhydrazone compound obtained can be directly used in the next step; Add copper fluoromethanesulfonate (1:9) to the reaction flask, add 10 mL of anhydrous toluene, add 2.4 mmol of vinyltributyltin under nitrogen protection, stir and react at room temperature for 1 hour, then raise the temperature to 110°C, and continue to stir and react for 15 hours , TLC detects that the reaction is complete, the obtained reaction mixture is filtered through diatomaceous earth, washed with ethyl acetate, the solvent is concentrated under vacuum and reduced pressure, and the crude product is separated by column chromatograph...
Embodiment 2
[0039] Preparation of compound 3b:
[0040] Weigh 5mmol of 2-(2-iodonaphthalene-1-yl)benzaldehyde in a reaction flask, add 30mL of ethanol, then add 6mmol of benzohydrazide, and stir the reaction at 80°C overnight. TLC detects that the reaction is complete. The reaction was cooled to room temperature, and the precipitated solid was filtered and washed with petroleum ether, and the obtained acylhydrazone compound could be used directly in the next step; Add copper (1:9) to the reaction flask, add 10mL of anhydrous toluene, add 2.4mmol of vinyltributyltin under nitrogen protection, stir and react at room temperature for 1 hour, then raise the temperature to 110°C, continue to stir and react for 15 hours, and detect the reaction by TLC Completely, the obtained reaction mixture is filtered through diatomaceous earth, washed with ethyl acetate, the solvent is concentrated under vacuum and reduced pressure, and the crude product is separated by column chromatography to obtain the pr...
Embodiment 3
[0044] Preparation of compound 3c:
[0045] Weigh 5mmol of 2-(2-iodo-1H-pyrrol-1-yl)benzaldehyde into a reaction flask, add 30mL of ethanol, and then add 6mmol of benzohydrazide, stir the reaction at 80°C overnight, and detect the reaction by TLC Complete, the reaction is cooled to room temperature, the precipitated solid is filtered, washed with petroleum ether, and the acylhydrazone compound obtained can be used directly in the next step; Add copper methanesulfonate (2:8) to the reaction flask, add 10 mL of anhydrous toluene, add 2.6 mmol of vinyltributyltin under nitrogen protection, stir and react at room temperature for 1 hour, then raise the temperature to 110°C, and continue to stir and react for 20 hours. TLC detected that the reaction was complete, and the obtained reaction mixture was filtered through diatomaceous earth, washed with ethyl acetate, the solvent was concentrated under vacuum and reduced pressure, and the crude product was separated by column chromatogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com