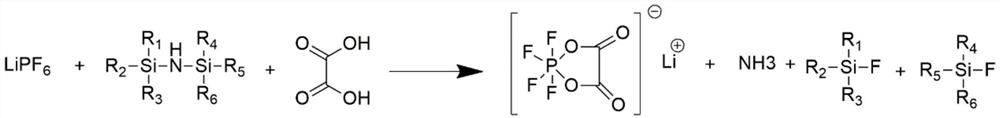
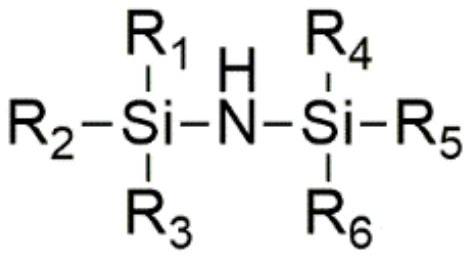Preparation method of lithium tetrafluoro (oxalato) phosphate and lithium difluoro (oxalato) phosphate
A technology of lithium difluorobis oxalate phosphate and lithium tetrafluorooxalate phosphate is applied in the field of lithium ion batteries, and can solve the problems of difficult control of chloride ion and acid value, high equipment requirements, hidden dangers and risks in safety and reliability, and the like, Achieve the effect of reducing equipment requirements, safe reaction conditions, and improving productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Under the protection of nitrogen, 18.08g of oxalic acid and 125.0g of diethyl carbonate were added to a dry 1000mL three-necked flask, stirred, and then 32.3g of hexamethyldisilazane was added, and stirred for 30min to make it evenly mixed. Preparation of lithium hexafluorophosphate solution: under the protection of nitrogen, take 38.0 g of lithium hexafluorophosphate and add in batches to 125.0 g of diethyl carbonate, and stir while adding to make it fully dissolved. Set the temperature to 50°C. After the flask reaches the set temperature, slowly add lithium hexafluorophosphate solution dropwise for 3 hours. After the dropwise addition, continue the heat preservation reaction for 2 hours, then lower the temperature to 30°C, use an oil pump to draw negative pressure, control the pressure in the flask to 1000 Pa, keep it for 2 hours, and then fill it with nitrogen to prepare for filtration. Filtrate under positive nitrogen pressure, and select 0.45um polypropylene membra...
Embodiment 2
[0030] Under the protection of nitrogen, add 40.7g of oxalic acid and 125.0g of diethyl carbonate into a dry 1000mL three-necked flask, stir, then add 80.7g of hexamethyldisilazane, stir for 30min to make it evenly mixed. Preparation of lithium hexafluorophosphate solution: under the protection of nitrogen, take 38.0 g of lithium hexafluorophosphate and add in batches to 125.0 g of diethyl carbonate, and stir while adding to make it fully dissolved. Set the temperature to 60°C. After the flask reaches the set temperature, slowly add lithium hexafluorophosphate solution dropwise for 3 hours. After the dropwise addition, continue the heat preservation reaction for 2 hours, then lower the temperature to 30°C, use an oil pump to draw negative pressure, control the pressure in the flask to 1000 Pa, keep it for 2 hours, and then fill it with nitrogen to prepare for filtration. Filtrate under positive nitrogen pressure, and select a 0.45um polypropylene membrane as a microporous filt...
Embodiment 3
[0032] Under the protection of nitrogen, add 22.6g oxalic acid and 125.0g ethylene glycol dimethyl ether into a dry 1000mL three-necked flask, stir, then add 40.4g hexamethyldisilazane, stir for 30min to make it evenly mixed. Preparation of lithium hexafluorophosphate solution: under the protection of nitrogen, take 38.0 g of lithium hexafluorophosphate and add in batches to 125.0 g of diethyl carbonate, and stir while adding to make it fully dissolved. The temperature was set at 75°C, and after the flask reached the set temperature, lithium hexafluorophosphate solution was slowly added dropwise for 4 hours. After the dropwise addition, continue the heat preservation reaction for 5 hours, then lower the temperature to 40°C, use an oil pump to draw negative pressure, control the pressure in the flask to 1000 Pa, keep it for 2 hours, and then fill it with nitrogen to prepare for filtration. Filtrate under positive nitrogen pressure, and select 0.45um polypropylene membrane as th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com