Protective cap for pre-filled syringe and manufacturing method of protective cap
A syringe and prefilling technology, which is applied in the direction of hypodermic injection devices, devices introduced into the body, needles, etc., can solve the problems of prefilled syringe performance impact, manpower, material and financial resources, etc., and achieve high cleanliness and radiation performance excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] A luer lock protective cap for prefilled syringes, prepared from the following raw materials in parts by mass: 50 parts of rubber A polyisoprene rubber, 50 parts of rubber B bromobutyl rubber; vulcanizing agent 2,5-dimethyl 0.8 parts of -2,5-bis(tert-butylperoxy)hexane, 0.4 parts of sulfur; 1.2 parts of co-curing agent trimethylolpropane trimethacrylate, 2 parts of magnesium oxide; 65 parts of filler calcium carbonate, 10 parts of white carbon black; 3 parts of pigment titanium dioxide, 0.3 parts of carbon black.
[0017] The manufacturing method is as follows:
[0018] (1) Compounding: Weigh each material according to the formula.
[0019] (2) Kneading: Knead and mix the weighed materials on the internal mixer and the open mixer respectively.
[0020] (3) Vulcanization: Vulcanize at 180°C for 7 minutes using a protective cap vulcanization mold.
[0021] (4) Punching: Punch and trim the vulcanized protective cap film on a punching machine.
[0022] (5) Cleaning and ...
Embodiment 2
[0026] A needle shield for prefilled syringes, prepared from the following raw materials in parts by mass: 70 parts of rubber A polyisoprene rubber; 30 parts of rubber B EPDM rubber; vulcanizing agent 1, 1-2 1 part of tert-butylperoxy-3,3,5-trimethylcyclohexane, 0.5 part of tert-octylphenol formaldehyde resin; 1.2 parts of triallyl cyanurate, 1 part of zinc oxide; filler carbonic acid Calcium 50 parts, kaolin 15 parts, white carbon black 10 parts; pigment titanium dioxide 3 parts, carbon black 0.3 parts.
[0027] Manufacturing method is the same as embodiment 1.
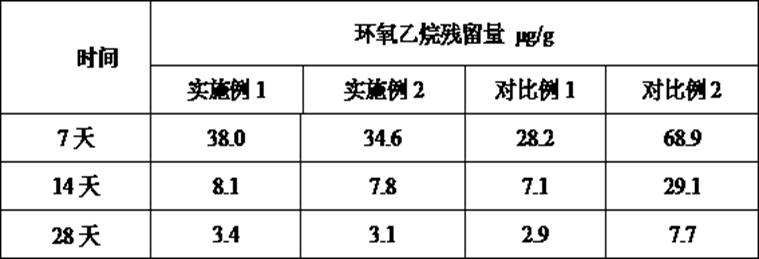
[0028] The reference standard "YBB00102004-2015 Polyisoprene Rubber Needle Protective Cap for Prefilled Syringes" of the piston product produced by the above method was tested, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com