A kind of albuterol sulfate sustained-release inhalation preparation and its production process
A technology for salbutamol sulfate and inhalation preparations is applied in respiratory diseases, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., to achieve the effects of improving heat resistance stability, improving dispersibility, and reducing agglomeration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
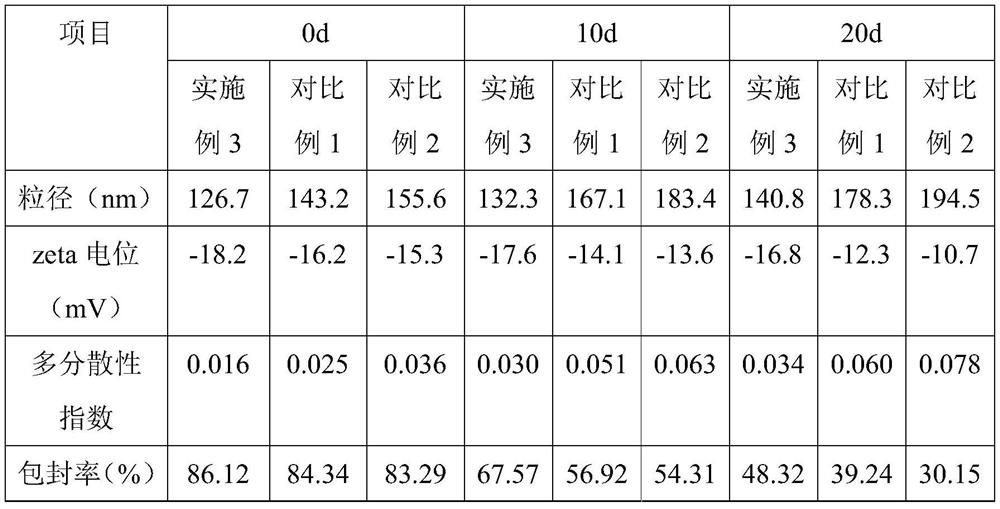
[0038] Embodiment 1, a kind of albuterol sulfate sustained-release inhalation preparation
[0039] The salbutamol sulfate sustained-release inhalation preparation includes the following components and parts by mass: 120 parts of salbutamol sulfate complex liquid, 88 parts of liposomes, 1 part of emulsifier, 0.5 part of stabilizer; the liposomes are composed of lecithin , glyceryl behenate and cholesterol are composed of 10:7:2 in mass ratio; the emulsifier is composed of sucrose fatty acid ester and sodium stearoyl lactylate in a mass ratio of 4:1; the stabilizer is composed of oleic acid and half milk Mannan is composed by mass ratio of 8:3.
[0040] Said salbutamol sulfate compound solution is made up of following components and mass parts thereof: 20 parts of salbutamol sulfate, 1 part of polyethylene glycol with molecular weight of 400, 0.2 part of polyvinylpyrrolidone with molecular weight of 58000, oligochitosan with molecular weight of 1000 1 part, 620 parts of water. ...
Embodiment 2
[0051] Embodiment 2, a kind of albuterol sulfate sustained-release inhalation preparation
[0052] The salbutamol sulfate sustained-release inhalation preparation includes the following components and parts by mass: 140 parts of salbutamol sulfate complex liquid, 105 parts of liposomes, 2 parts of emulsifiers, 1.2 parts of stabilizers; the liposomes are composed of lecithin , glyceryl behenate and cholesterol are formed by mass ratio 13:4:1; Described emulsifier is made up of alkyl polyglucoside and sorbitan oleic acid ester by mass ratio 3:2; Described stabilizing agent is made up of oleic acid and The galactomannans are composed at a mass ratio of 12:1.
[0053] Said salbutamol sulfate complex liquid is made up of following components and mass parts thereof: 25 parts of salbutamol sulfate, 2 parts of polyethylene glycols with molecular weight of 400, 0.5 part of polyvinylpyrrolidone with molecular weight of 58000, chitosan oligosaccharide with molecular weight of 1000 3 par...
Embodiment 3
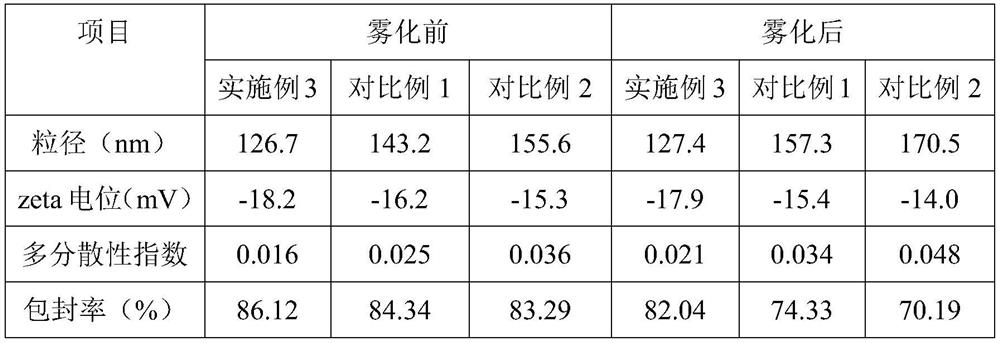
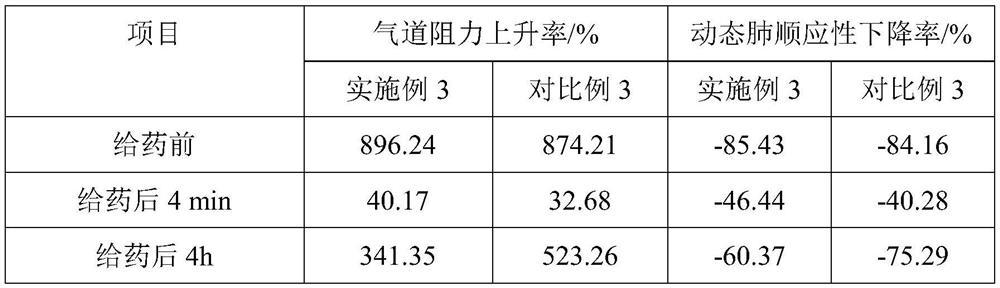
[0064] Embodiment 3, a kind of albuterol sulfate sustained-release inhalation preparation
[0065] The salbutamol sulfate sustained-release inhalation preparation includes the following components and parts by mass: 130 parts of salbutamol sulfate complex solution, 95 parts of liposomes, 1.6 parts of emulsifier, 0.9 parts of stabilizer; the liposomes are composed of lecithin , glyceryl behenate and cholesterol are composed of 12:5:1 by mass ratio; the emulsifier is composed of sucrose fatty acid ester and alkyl polyglucoside by mass ratio of 2:5; the stabilizer is composed of oleic acid and galactose Mannan is composed by mass ratio of 10:3.
[0066] Said salbutamol sulfate compound solution is made up of following components and mass parts thereof: 22 parts of salbutamol sulfate, 1.4 parts of polyethylene glycol with molecular weight of 400, 0.3 part of polyvinylpyrrolidone with molecular weight of 58000, chitosan oligosaccharide with molecular weight of 1000 2 parts, 660 pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com