Peptide compound as well as preparation method and application thereof
A compound and target compound technology, applied in the field of peptide compounds and their preparation, can solve problems such as poor quality, achieve strong inhibitory activity and promote cell apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
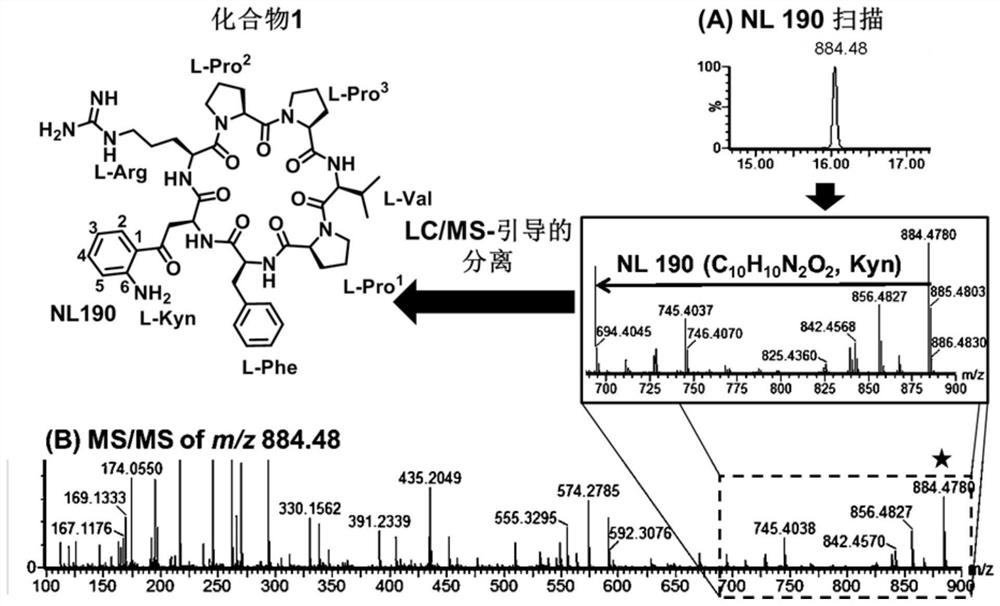
[0063] Embodiment 1 finds and extracts natural compound 1 (C from sponge) 45 h 61 N 11 o 8 )
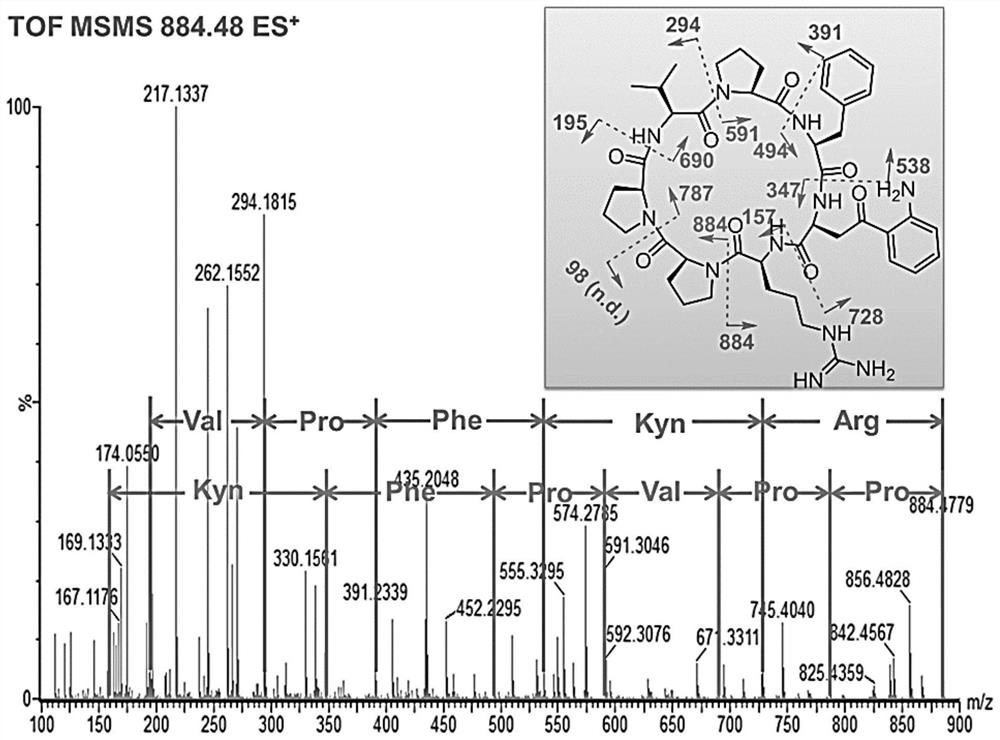
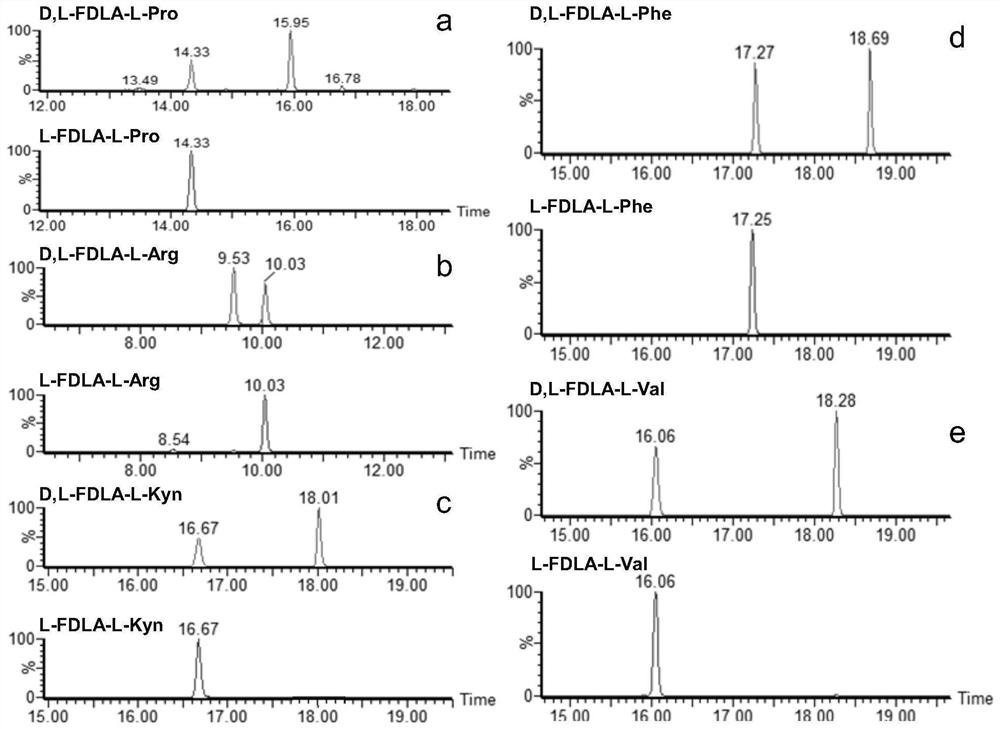
[0064] From the mass spectrometry fragmentation mechanism of peptide compounds, it is found that the secondary fragment ions formed after the collision-induced dissociation of cyclic peptides are often caused by the loss of certain neutral molecular fragments. Most of these neutral molecules are the basic units of the cyclic peptide— - Each amino acid residue. According to this feature, the analysis of secondary MS found that the cyclic peptide containing kynurenine can be quickly searched through the parent ion with a poor mass of 190Da among the fragment ions. The crude fraction of the brown flat sponge Phakellia fusca (belonging to the class Demospongiae, order Halichondrid, family Axinellidae, genus Phakellia) collected from the waters near the Xisha Islands in China was scanned by neutral loss mass spectrometry , from which the precursor ion was found to be m / z884.48[M+H] ...
Embodiment 2
[0094] Embodiment 2 prepares compound 1 of the present invention by synthesis
[0095] Synthetic route of compound 1
[0096]
[0097] The synthesis steps are as follows:
[0098] Loading of the first amino acid into 2-CTC resin: 2-CTC resin (100 mg, loading: 1.0 mmol / g) was swelled in a disposable container (TORIVQ) with 2 mL of anhydrous DCM for 20 minutes. Fmoc-Pro-OH (2.0 equiv) and DIEA (4.0 equiv) in DCM were added and the reaction vessel was shaken on a vortex at room temperature for 1 hour. Add 200 µL of MeOH to the reaction mixture and spin the resin for 15 min. The resin was filtered and washed with anhydrous DCM (3 mL×5 times, 1 min / time), 1:1 DCM / MeOH (v / v) (3 mL×5 times, 1 min / time) and MeOH (3 mL×2 times, 1 min / time) times) washing.
[0099] Fmoc deprotection: at room temperature, use 3 mL of 20% piperidine in DMF solution for Fmoc deprotection for 20 minutes, and then wash the resin with DMF (3 mL×2 times, 1 minute / time).
Embodiment 3
[0107] Embodiment 3 The in vitro activity experiment of compound 1 of the present invention
[0108] Cytotoxic activity test: the compound 1 of the present invention was tested for its cytotoxic activity against six tumor cell lines (MCF-7, HeLa, NCI-H460, SW480, PC9 and HepG2) and one normal cell line (H9C2). The sample was dissolved in DMSO and stored at low temperature. The concentration of DMSO in the final system was controlled within the range that did not affect the detection activity, and the working concentration was 1-100 μg / mL by doubling dilution. Take the cells in the logarithmic growth phase and make a single cell suspension 1×10 6 per mL, the suspension was added to a 96-well plate, and 100 μL was added to each well. at 5% CO 2 After culturing in a 37°C incubator for 24 hours, each concentration of the test drug was added to make the final concentrations respectively 100, 50, 25, 12.5, and 6.25 μg / mL. Three replicate holes were set up for each sample, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com