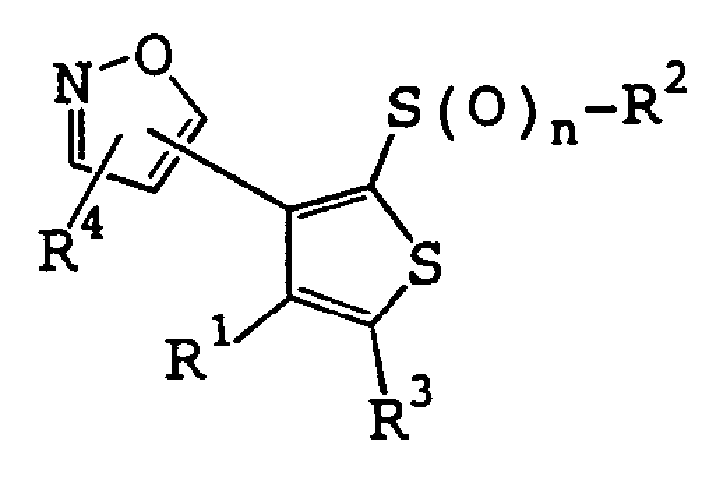
Substitute ixoxazolylthiophene compounds
A technology of isoxazolylthiophene and compounds, which is applied in the field of substituted isoxazolylthiophene compounds, and can solve the problems of undocumented compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 3,4'-Dimethyl-4-(isoxazol-5-yl)-5-(methylthio)thiophene-2-carboxamide
[0059] a) Ethyl 3-methyl-5-methylthio-4-propionylthiophene-2-carboxylate
[0060] Add 85% aqueous solution (8ml) containing potassium hydroxide (13.6g, 175.2mmol) dropwise to a solution containing 2,4-hexanedione (10.0g, 87.6mmol) in dimethyl sulfoxide (88ml) under ice cooling ) and carbon disulfide (7.3 g, 96.4 mmol), and the mixture was stirred at this temperature for 30 minutes. Next, a solution containing ethyl bromoacetate (13.2 g, 78.8 mmol) in dimethylsulfoxide (8 ml) was added dropwise under ice-cooling, and the mixture was stirred at this temperature for 30 minutes. Iodomethane (12.4 g, 87.6 mmol) was then added, and the mixture was stirred for a further 20 minutes. The reaction solution was extracted with ethyl acetate, and the organic layer was washed (successively with water and saturated aqueous sodium chloride), dried (over anhydrous magnesium sulfate), filtered and concentrated und...
Embodiment 2
[0071] 3-Ethyl-4-(4-methylisoxazol-5-yl)-5-(methylthio)thiophene-2-carbonitrile
[0072] a) 3-Ethyl-5-methylthio-4-propionylthiophene-2-carbonitrile
[0073] To a solution containing 2,4-heptanedione (10.0 g, 78.0 mmol) in dimethylsulfoxide (100 ml), an 85% aqueous solution containing potassium hydroxide (10.3 g, 156.0 mmol) was successively added dropwise under ice-cooling ( 10ml) and carbon disulfide (5.9g, 78.0mmol), then the mixture was stirred at this temperature for 30 minutes. Next, a solution containing chloroacetonitrile (5.3 g, 70.2 mmol) in dimethylsulfoxide (10 ml) was added dropwise over 5 minutes under ice-cooling, and the mixture was stirred at this temperature for 20 minutes. Potassium carbonate (10.8 g, 78.0 mmol) and iodomethane (12.2 g, 85.8 mmol) were then added, and the mixture was stirred for a further 30 minutes. The reaction solution was extracted with ethyl acetate, and the organic layer was washed (successively with water and saturated aqueous sod...
Embodiment 3
[0079] 3-Ethyl-4-(4-methylisoxazol-5-yl)-5-(methylthio)thiophene-2-carboxamide
[0080] To 3-ethyl-4-(4-methylisoxazol-5-yl)-5-(methylthio)thiophene-2-carbonitrile (0.87g, 3.3mmol) was added concentrated sulfuric acid (11ml) and The mixture was heated at 60°C for 1.5 hours. The reaction solution was cooled to room temperature, ice was added, and the mixture was extracted with ethyl acetate. The organic layer was washed (successively with water, saturated aqueous sodium bicarbonate and saturated aqueous sodium chloride), dried (over anhydrous magnesium sulfate), and concentrated under reduced pressure. The residue thus obtained was purified by silica gel column chromatography (developing solvent: chloroform / ethyl acetate=1:0-3:1), and recrystallized in ether to obtain 3-ethyl-4-(4-methyl (isoxazol-5-yl)-5-(methylthio)thiophene-2-carboxamide (0.39 g, 42%) as colorless crystals.
[0081] Melting point: 93.5-94.5°C
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com