Method for preparing medical adhesive from alpha-alkyl cyanoacrylate monomer
A technology of alkyl cyanoacrylate and monomer, which is applied in the field of preparing medical adhesive from α-alkyl cyanoacrylate monomer, can solve the problem of reducing the bonding ability of α-alkyl cyanoacrylate and medical , reduce the reaction speed of the reagents, etc., to achieve the effect of increasing the reaction rate, improving the thermal conductivity, and improving the efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
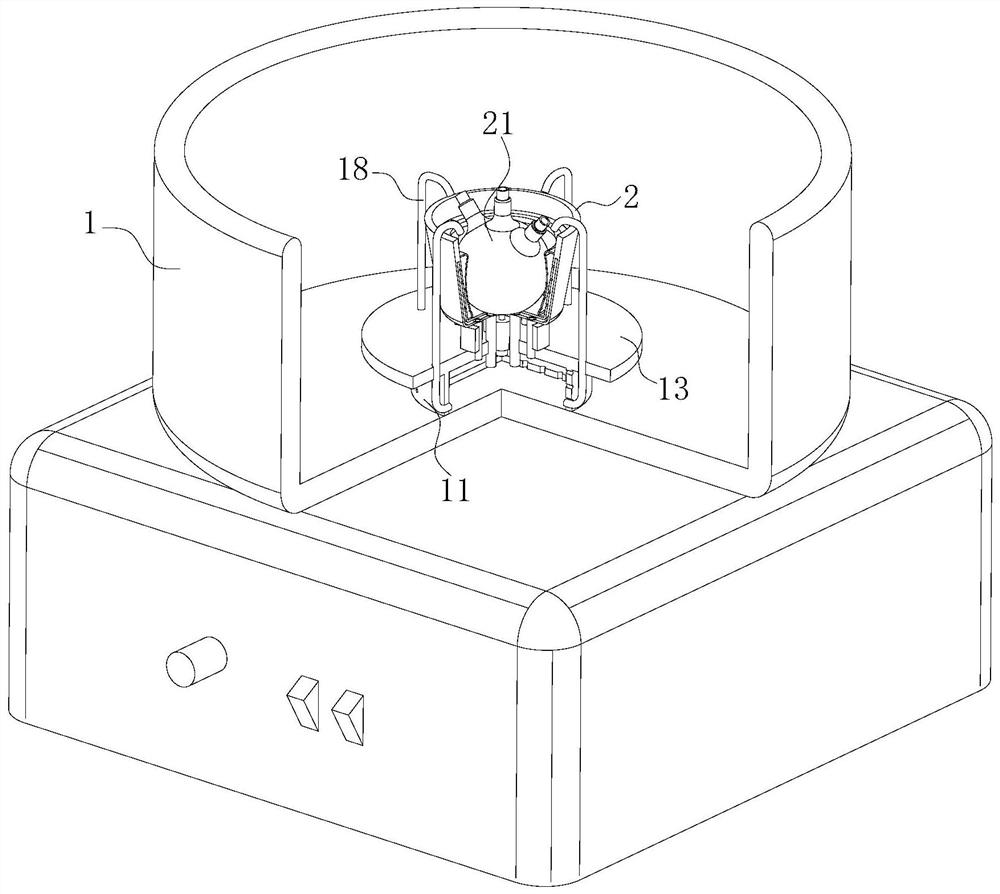
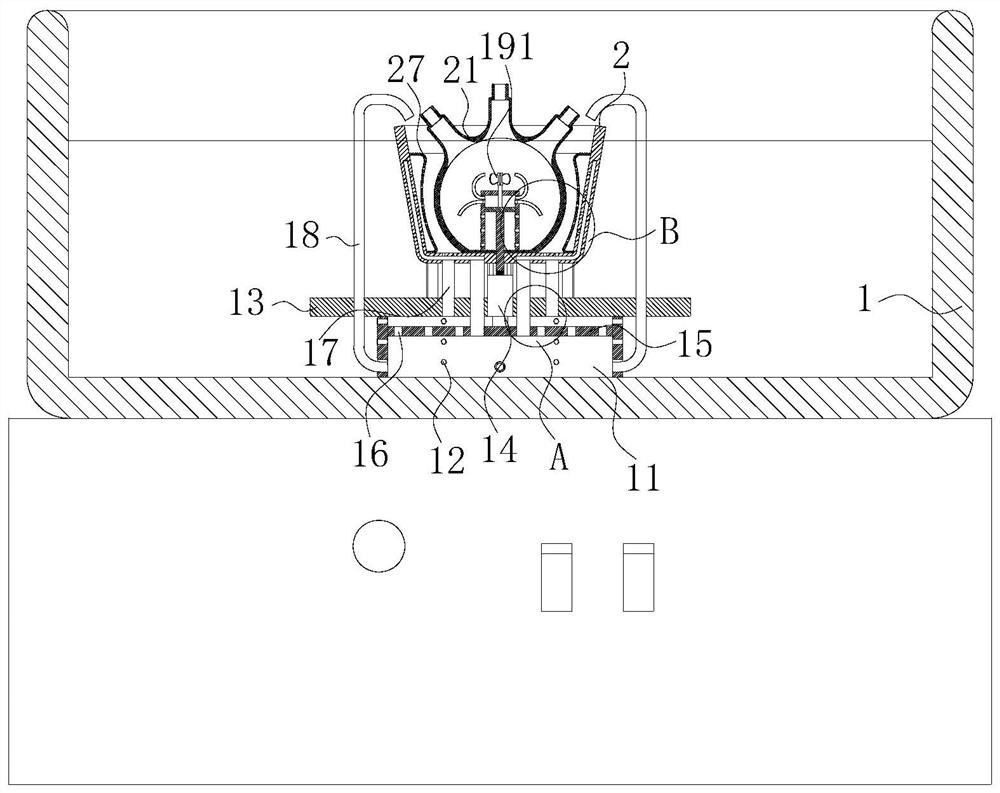
[0047] As an embodiment of the present invention, an arc-shaped bag 27 is fixedly connected to the inner surface of the oil bath bin 2 in the cavity of the oil bath bin 2, and the arc-shaped bag 27 is made of heat-conducting silica gel material; the arc-shaped bag 27 Design of the third through hole 23 of the wrapping part; uniformly arranged spray holes 28 are opened in the inner wall of the arc-shaped bag 27;
[0048] During work, since the arc-shaped layer partly wrapped on the inner surface of the oil bath warehouse 2 is designed to wrap the third through-hole 23, the oil flowing out in the third through-hole 23 can flow into the arc-shaped bag 27, when the oil in the arc-shaped bag 27 When the oil increases, it will expand, and when the arc-shaped bag 27 expands, it can be attached to the outer surface of the three-necked flask 21. In this process, the three-necked flask 21 can be heated evenly, and at the same time, the diffusion of the stock solution in the three-necked ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com