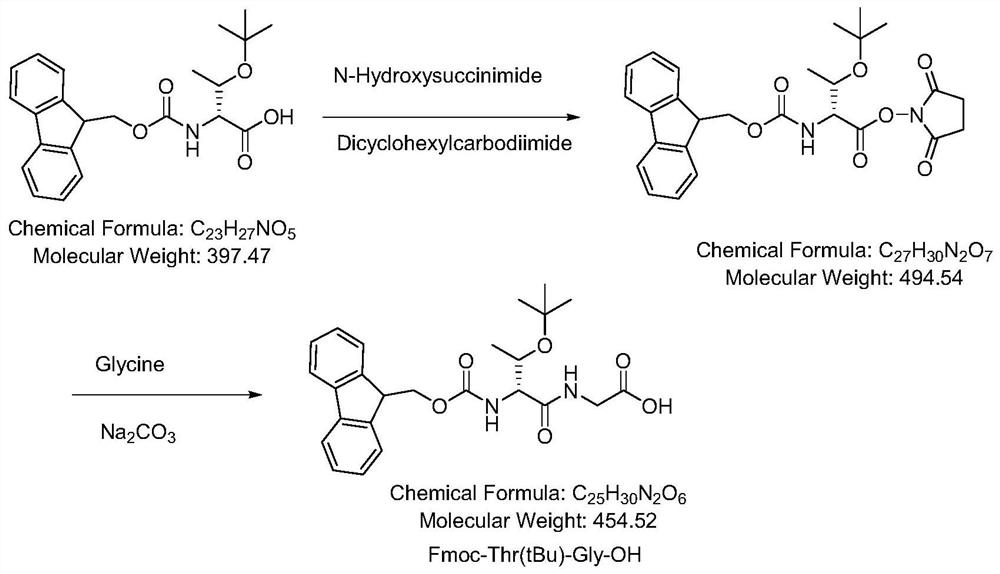
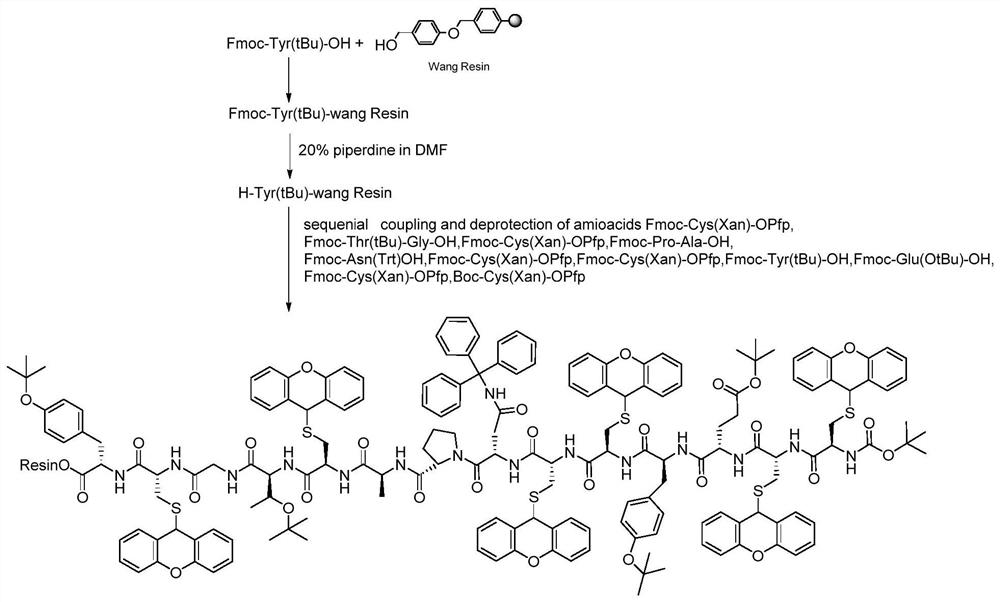
Synthesis method of linaclotide
A technology of linaclotide and synthetic method, which is applied in the field of drug synthesis, can solve problems such as breaking disulfide bonds, and achieve the effects of simple process operation, high coupling efficiency, and prevention of β-sheet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
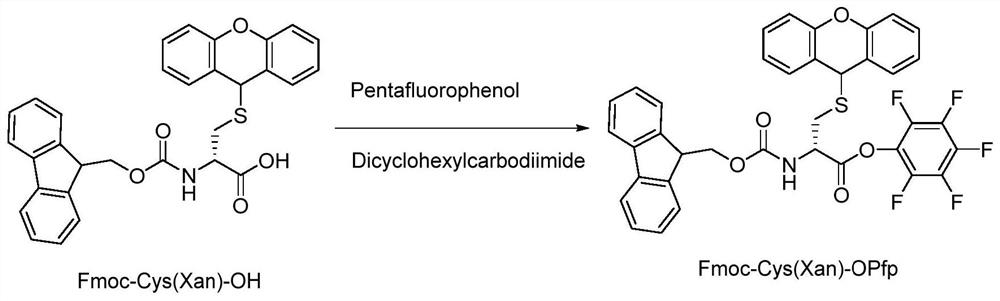
[0084] 1. Synthesis of Fmoc-Cys(Xan)-OPfp
[0085] Fmoc-Cys(Xan)-OH (100 g, 190 mmol) was weighed and added into a three-necked flask containing 700 mL of tetrahydrofuran. The mixture was stirred at 25±2°C for 5 minutes. Then 42.18 g (229.2 mmol) of pentafluorophenol dissolved in 100.0 mL of THF was added thereto and stirred for 5-10 minutes. In another round bottom flask, 47.3 g of dicyclohexylcarbodiimide (229.2 mmol) was dissolved in 200 ml of tetrahydrofuran. This solution was slowly added dropwise (30-45min) to the above-mentioned Fmoc-Cys(Xan)-OH tetrahydrofuran solution at 25±2°C. After the addition, it was stirred at the same temperature for 3-4h, and the complete reaction of the raw materials was monitored by thin layer chromatography (TLC). The reaction process is as figure 1 shown.
[0086] The reaction solution was filtered to remove urea, and the solvent tetrahydrofuran was completely removed by a rotary evaporator to obtain a colloidal solid product. Isopro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com