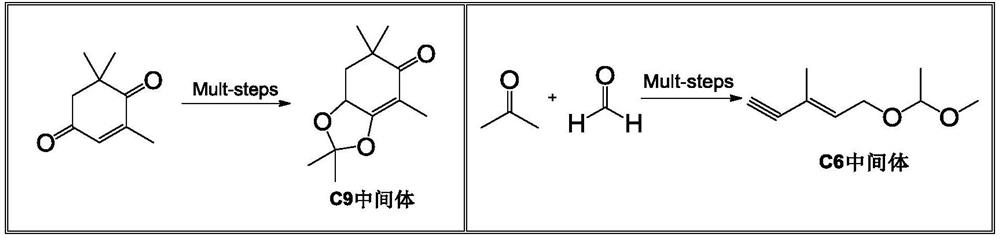
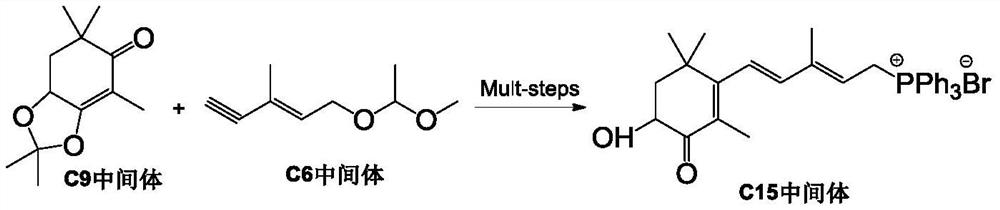
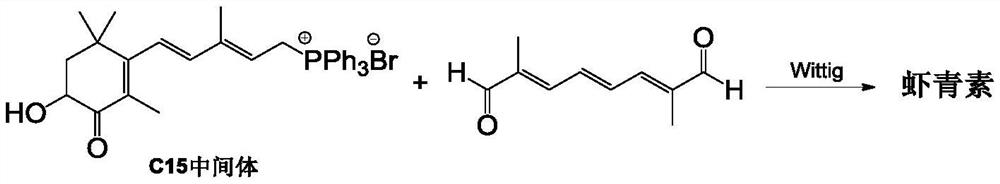
Method for preparing astaxanthin by oxidizing canthaxanthin
A technology of canthaxanthin and astaxanthin, applied in the direction of organic chemistry, can solve the problems of high price, high risk, and easy decomposition of n-butyl lithium, and achieve increased production safety, short process route, and less reaction materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Under the protection of nitrogen, put 5.64g (10mmol) of canthaxanthin into a 500ml three-necked flask, add 180ml of methanol to dissolve, then add 3.30g (15mmol) of oxidant iodosobenzene, lower the reaction temperature to 10°C, stir and mix evenly, Dissolve 0.20 g (5.0 mmol) of catalyst sodium hydroxide in 20 ml of methanol, and slowly add it dropwise to the reaction system within 30 minutes. Methanol was removed, water and dichloromethane were added for washing, and the layers were separated to obtain an organic phase containing crude dialkoxy ketal.
[0044] The above-mentioned organic phase was transferred to a 500ml three-necked flask, and 1.96g (1.0mmol) of 5wt% sulfuric acid aqueous solution was added, and the reaction was stirred at room temperature. After reacting for 5h, adding a 5wt% aqueous solution containing 2.0mmol sodium bicarbonate was quenched. The reaction was washed with water three times, the organic phase was separated, dried over anhydrous magnesiu...
Embodiment 2
[0047]Under nitrogen protection, put 5.64g (10mmol) of canthaxanthin into a 500ml three-necked flask, add 180ml of methanol to dissolve, then add 3.99g (12mmol) of iodobenzene acetate as an oxidant, lower the reaction temperature to 10°C, stir and mix evenly, and Catalyst potassium hydroxide 0.28g (5.0mmol) was dissolved in 20ml of methanol, slowly added dropwise to the reaction system within 30 minutes, after the dropwise addition was completed, the temperature was raised to room temperature, the reaction pressure was normal pressure, the reaction time was 12h, and the reaction ended Afterwards, methanol was removed under reduced pressure, water and dichloromethane were added for washing, and the layers were separated to obtain an organic phase containing crude dialkoxy ketal.
[0048] The above-mentioned organic phase was transferred to a 500ml three-necked flask, and 1.96g (1.0mmol) of 5wt% sulfuric acid aqueous solution was added, and the reaction was stirred at room temper...
Embodiment 3
[0050] Under the protection of nitrogen, put 5.64g (10mmol) of canthaxanthin into a 500ml three-necked flask, add 180ml of methanol to dissolve, then add 3.30g (15mmol) of oxidant iodosobenzene, lower the reaction temperature to 0°C, stir and mix evenly, Dissolve 0.20 g (5.0 mmol) of catalyst sodium hydroxide in 20 ml of methanol, and slowly add it dropwise to the reaction system within 30 minutes. After the dropwise addition is completed, the temperature is raised to 40° C., the reaction pressure is normal pressure, and the reaction time is 10 hours. After the reaction, methanol was removed under reduced pressure, water and dichloromethane were added for washing, and liquid separation was carried out to obtain an organic phase containing crude dialkoxy ketal.
[0051] The above-mentioned organic phase was transferred to a 500ml three-necked flask, and 1.96g (1.0mmol) of 5wt% sulfuric acid aqueous solution was added, and the reaction was stirred at room temperature. After react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com