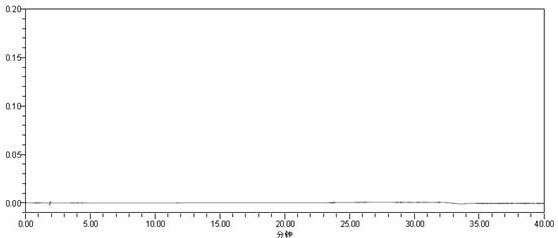
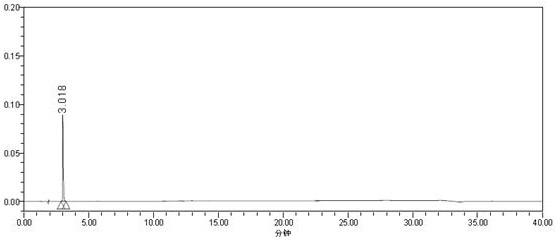
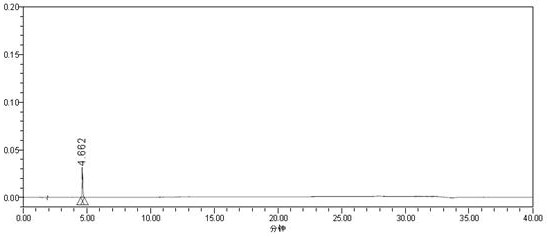
Method for detecting carmustat mesylate related substances by adopting high performance liquid chromatography and application
A camostat mesylate and high performance liquid chromatography technology, applied in the field of medicine, can solve the problems of complex impurity spectrum and poor stability of camostat mesylate, and achieve high sensitivity, specificity and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1) Experimental conditions
[0048] Agilent1260-DAD high performance liquid chromatograph;
[0049] Chromatographic column: Waters Xbridge RP18 4.6×150mm 3.5μm;
[0050] Flow rate: 1.0mL / min;
[0051] Column temperature: 35°C;
[0052] Injection volume: 10 μL;
[0053] Sample concentration: 1mg / ml;
[0054] Diluent: 90% acetonitrile aqueous solution;
[0055] Detection wavelength: 230nm and 265nm;
[0056]Mobile phase A: 0.075% (mass fraction) of sodium heptanesulfonate, and 0.1% (volume fraction) of trifluoroacetic acid.
[0057] Mobile Phase B: Acetonitrile
[0058]
[0059] 2) Solution preparation
[0060] Impurity reference substance stock solution: take appropriate amounts of p-hydroxyphenylacetic acid reference substance, intermediate 3 reference substance, intermediate 5 reference substance, impurity C reference substance and impurity J reference substance, weigh them accurately, dissolve with diluent and dilute quantitatively Make a solution containi...
Embodiment 2
[0069] Experimental conditions:
[0070] Agilent1260-DAD high performance liquid chromatograph;
[0071] Chromatographic column: Waters Xbridge RP18 4.6×150mm 3.5μm;
[0072] Flow rate: 1.0mL / min;
[0073] Detection wavelength: 230nm and 265nm;
[0074] Column temperature: 35°C;
[0075] Injection volume: 10 μL;
[0076] Sample concentration: 1mg / ml;
[0077] Diluent: 90% acetonitrile aqueous solution;
[0078] Detection wavelength: 230nm and 265nm;
[0079] Mobile phase A: 0.075% (mass fraction) of sodium heptanesulfonate, and 0.05% (volume fraction) of trifluoroacetic acid.
[0080] Mobile Phase B: Acetonitrile
[0081]
[0082] 2) Solution preparation
[0083] Impurity reference substance stock solution: take appropriate amounts of p-hydroxyphenylacetic acid reference substance, intermediate 3 reference substance, intermediate 5 reference substance, impurity C reference substance and impurity J reference substance, weigh them accurately, dissolve with diluent an...
Embodiment 3
[0092] Experimental conditions:
[0093] Agilent1260-DAD high performance liquid chromatograph;
[0094] Chromatographic column: Waters Xbridge RP18 4.6×150mm 3.5μm;
[0095] Flow rate: 0.8mL / min;
[0096] Detection wavelength: 230nm and 265nm;
[0097] Column temperature: 35°C;
[0098] Injection volume: 10 μL;
[0099] Sample concentration: 1mg / ml;
[0100] Diluent: 90% acetonitrile aqueous solution;
[0101] Detection wavelength: 230nm and 265nm;
[0102] Mobile phase A: 0.075% (mass fraction) of sodium heptanesulfonate, and 0.10% (volume fraction) of trifluoroacetic acid.
[0103] Mobile Phase B: Acetonitrile
[0104]
[0105] 2) Solution preparation
[0106] Impurity reference substance stock solution: take appropriate amounts of p-hydroxyphenylacetic acid reference substance, intermediate 3 reference substance, intermediate 5 reference substance, impurity C reference substance and impurity J reference substance, weigh them accurately, dissolve with diluent an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com