Flame retardant containing aromatic phosphoric acid ester, and thermoplastic resin composition containing same
一种芳香族磷酸酯、热塑性树脂的技术,应用在防火涂料等方向,能够解决热流动性难点、不充分等问题,达到改善热流动性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0127] The present invention will be more specifically described by the following synthesis examples and test examples (Examples and Comparative Examples), but the scope of the present invention is not limited to the examples in these test examples.
[0128] [Identification of aromatic phosphates]
[0129] Using the following measuring devices and conditions, by 1 The aromatic phosphoric acid ester obtained in the synthesis example was identified by H-NMR.
[0130] ・Measuring device: manufactured by JEOL Ltd., 1 H-NMR device (model: JNM-ECS-400)
[0131] Solvent: CDCl 3
[0132] [Evaluation of aromatic phosphate]
[0133] The aromatic phosphoric acid ester obtained in the synthesis example and the known aromatic phosphoric acid ester used in the comparative example were evaluated by the following method.
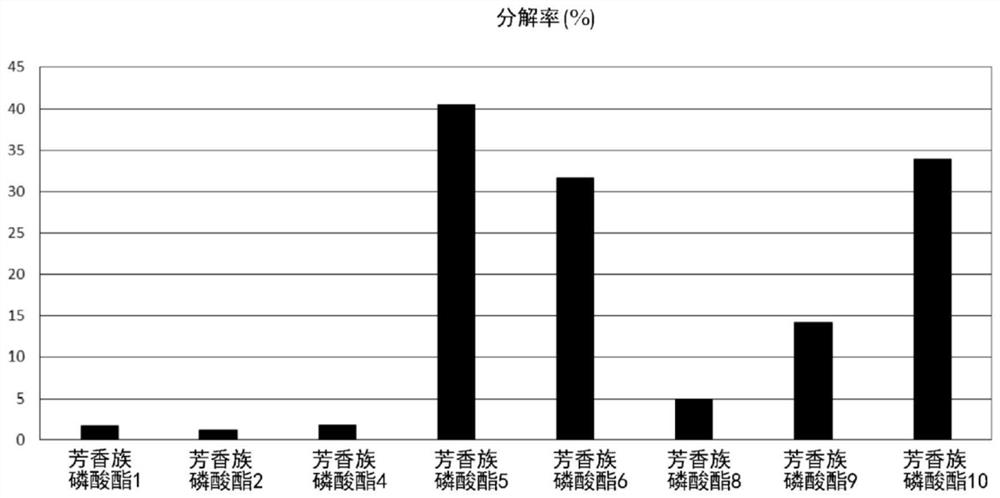
[0134] (hydrolysis resistance)
[0135] Hydrolysis resistance was evaluated based on the decomposition rate (%) obtained by the hydrolysis test (pressure cooker test)...
Synthetic example 1
[0196] (Synthesis example 1) Synthesis of di(xylyl) chlorophosphate (DXPC)
[0197] 767 g of phosphorus oxychloride, 1,200 g of 2,6-xylenol, and 140 g of xylene as a solvent were filled into a 2-liter four-necked flask equipped with a stirrer, a thermometer, and a hydrochloric acid recovery device (a condenser connected to a water scrubber). and 6.2 g of magnesium chloride as a catalyst.
[0198] While stirring the obtained mixed solution, it was gradually heated up to a temperature of 160°C over about 3 hours to allow a reaction to occur, and the generated hydrogen chloride (hydrochloric acid gas) was recovered by a water scrubber. Thereafter, at the temperature (160° C.), the pressure in the flask was gradually reduced to 20 kPa, and xylene, unreacted phosphorus oxychloride, 2,6-xylenol, and by-produced hydrogen chloride were removed to obtain 1700 g of a reaction mixture containing di(xylyl chlorophosphate) of the following structural formula as a main component. In addit...
Synthetic example 2
[0200] (Synthesis Example 2) Synthesis of Aromatic Phosphate 1
[0201] 460 g of di(xylyl chlorophosphate) obtained in Synthesis Example 1, 178 g of bisphenol 1, 540 g of toluene as a solvent, and tetrahydrofuran were charged into a four-necked flask with a capacity of 2 liters equipped with a stirrer, a thermometer, a dropping funnel, and a condenser. 140g. In addition, 151 g of triethylamine as a hydrogen halide scavenger was filled in the dropping funnel.
[0202] The mixed solution in the four-necked flask was heated to a temperature of 65° C. while stirring, and triethylamine in the dropping funnel was added dropwise over 1 hour and 30 minutes while maintaining the temperature (65° C.). After completion of the dropwise addition, stirring was carried out at the temperature (65° C.) for 2 hours to obtain a reaction product.
[0203] The obtained reaction product was washed with dilute hydrochloric acid and water, neutralized and washed with an aqueous sodium hydroxide sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com