Hydroxyapatite composite material with antibacterial function as well as preparation method and application thereof
A technology of hydroxyapatite and composite materials, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, applications, etc., and can solve problems such as unreported research on iodine-modified hydroxyapatite.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
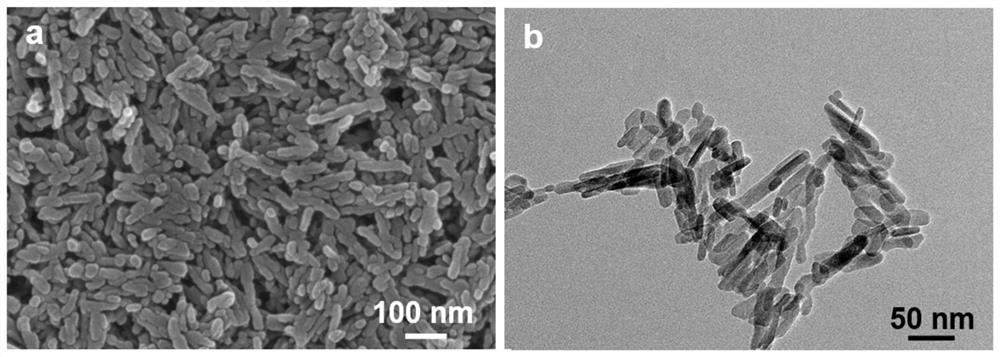
[0074] (1) Hydroxyapatite is purchased or prepared by chemical precipitation. The preparation method is: weigh 13.875g of anhydrous calcium chloride and dissolve it in 750mL of water, adjust the pH to 9.5 with ammonia water, stir for 30min, and add 250mL of 0.3 M(NH 4 ) 2 HPO 4 Aqueous solution, the pH of the solution is controlled to be 9-10 during the dropping process, then stirred and reacted at 75°C for 12-24 hours, centrifuged, washed alternately with water and ethanol for several times, and dried to obtain the following: figure 1 Hydroxyapatite shown.
[0075] (2) Ultrasonic disperse 5g of hydroxyapatite in 100mL of ethanol, add ammonia water to adjust the pH to 10, add 3g of 3-aminopropyltriethoxysilane, stir at 300rpm and reflux at 75°C for 24h, centrifuge, wash with ethanol Washed twice, dried in vacuum to obtain amino-functionalized hydroxyapatite.
[0076] (3) Dissolve 10g of 6-O-carboxymethyl chitosan (average weight-average molecular weight of 150,000g / mol, de...
Embodiment 2
[0084]Repeat steps (1)(2)(3) in Example 1 to prepare amino-functionalized hydroxyapatite and quaternized carboxymethyl chitosan, the difference is only to select the 6- O-carboxymethyl chitosan (deacetylation degree ≥ 85%, substitution degree ≥ 80%).
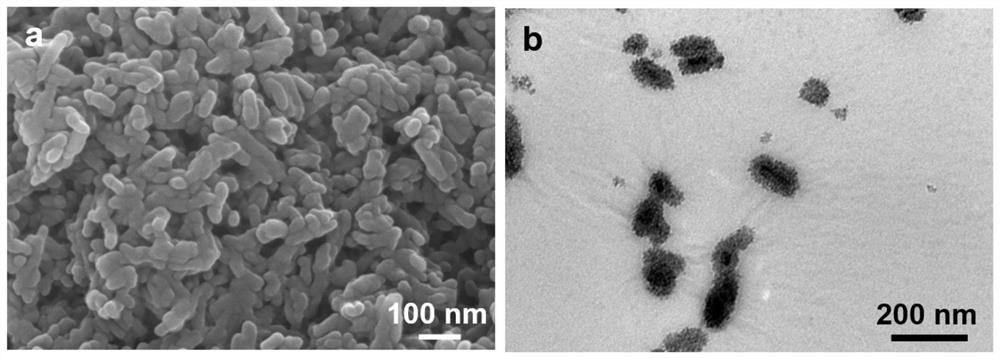
[0085] (4) Ultrasonic disperse 1g of amino-functionalized hydroxyapatite in 130mL of water, add 0.65g of quaternized carboxymethyl chitosan to completely dissolve, mix well, add 6.85mL of 100 / 50mM EDC / NHS mixture drop by drop Add in the above solution, the final concentration is 5.0 / 2.5mM, cross-link at room temperature for 24h, the cross-linked solution is centrifuged and washed, and freeze-dried to obtain the surface grafted quaternized carboxymethyl chitosan material (HA -g-GCMC).
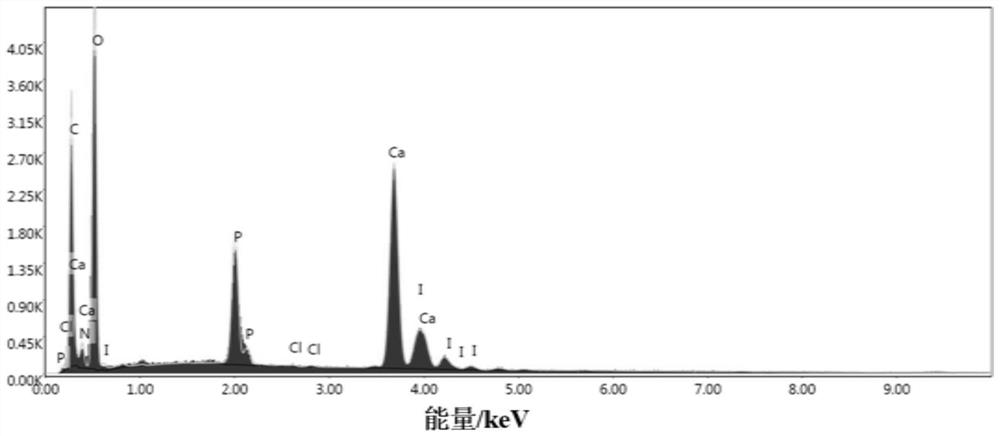
[0086] (5) Immerse the obtained HA-g-GCMC in 50mL of 0.01g / mL iodine / ethanol / water (volume ratio of ethanol to water is 1:3), stir at room temperature for 5h, wash and freeze-dry to obtain antibacterial hydroxyphosphorus Gray stone composite mat...
Embodiment 3
[0089] Repeat steps (1)(2)(3) in Example 1 to prepare amino functionalized hydroxyapatite and quaternized carboxymethyl chitosan, the difference is only that the selection of average weight average molecular weight is 1000g / mol of 6- O-carboxymethyl chitosan (deacetylation degree ≥ 85%, substitution degree ≥ 80%).
[0090] (4) Ultrasonic disperse 1g of amino-functionalized hydroxyapatite in 300mL of water, add 1.5g of quaternized carboxymethyl chitosan to dissolve completely, mix well, add 15.80mL of 200 / 100mM EDC / NHS mixture drop by drop Add in the above solution, the final concentration is 10.0 / 5.0mM, cross-linking at room temperature for 24h, the cross-linking solution, centrifugal washing, freeze-drying and drying to obtain hydroxyapatite surface grafted quaternized carboxymethyl chitosan material ( HA-g-GCMC).
[0091] (5) Immerse the obtained HA-g-GCMC in 80mL of 0.01g / mL iodine / ethanol / water (volume ratio of ethanol to water is 1:1), stir at room temperature for 5h, wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Average weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com