A-pi-D-pi-A type BODIPY small molecular photovoltaic material and preparation method and application thereof
A small molecule compound, selected technology, applied in photovoltaic power generation, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problems of lack of π bridge system research, few types of BODIPY conjugated small molecules, and low device efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] This example discloses the synthesis of compound Q1, the specific synthesis steps are as follows:
[0093] Under nitrogen, the compound ABr (0.720g, 1.52mmol), tetrakis (triphenylphosphine) palladium (26mg, 0.023mmol) was dissolved in 15mL of toluene, and then containing 9-octyl-2,7-bis{ A solution of 5-(tributyltinyl)-thiophen-2-yl}carbazole (0.409g, 0.40mmol) in 15mL of toluene was injected into the reaction system and refluxed at 110°C for 48h. The end of the reaction was detected by TLC, and the solvent in the reaction solution was removed. The crude product was separated by column chromatography with a mixture of petroleum ether / dichloromethane (2:1 by volume), and finally 0.168 g of a dark green solid was obtained, with a yield of 34%.
[0094] The reaction formula of preparing above-mentioned compound Q1 is as follows:
[0095]
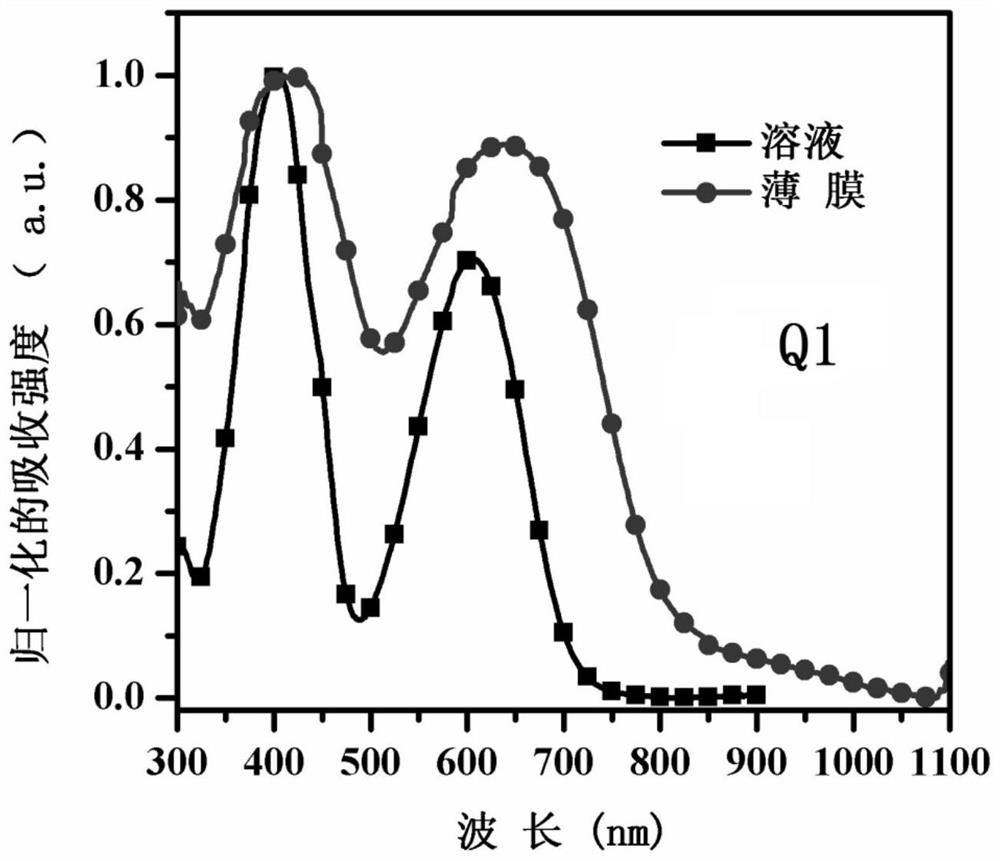
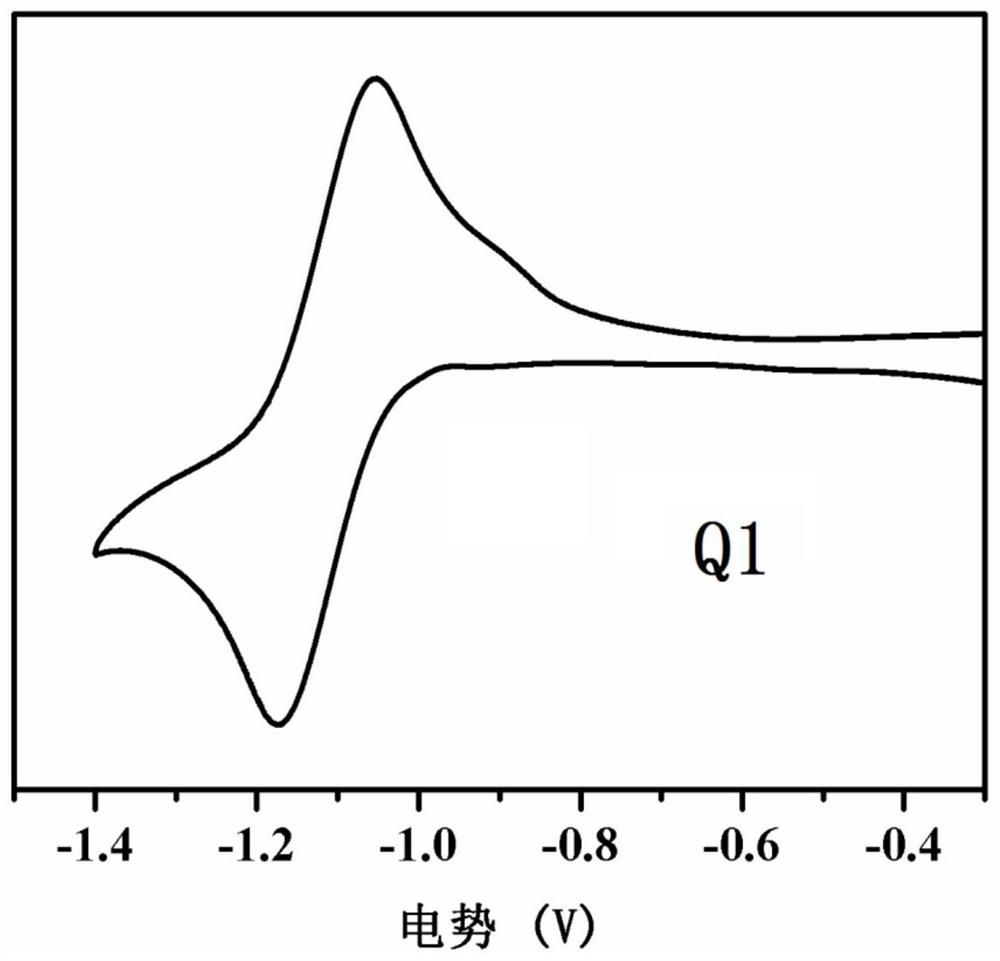
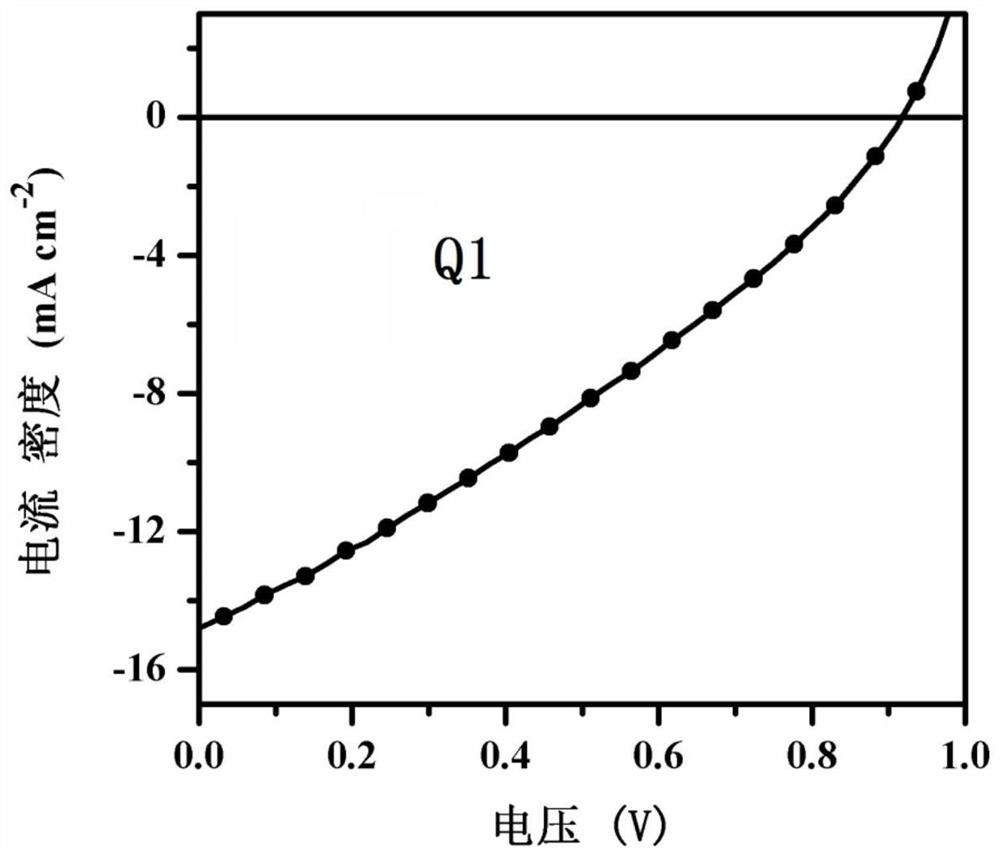
[0096] see figure 1 , is the normalized UV-Vis spectrum absorption figure of compound Q1 in chloroform solution and film-forming st...
Embodiment 2
[0100] This embodiment discloses the synthesis of compound Q2, and the specific synthesis steps are as follows:
[0101] Under nitrogen, the compound ABr (0.948g, 2.00mmol), tetrakis (triphenylphosphine) palladium (46mg, 0.04mmol) was dissolved in 20mL of toluene, and then containing 5,11-dioctyl-2,8 - Inject 20 mL of toluene solution of bis{5-(tributyltin)-thiophen-2-yl}indole[3,2-b]carbazole (0.819g, 0.67mmol) into the reaction system, and reflux at 110°C for 48h . The end of the reaction was detected by TLC, and the solvent in the reaction solution was removed. The crude product was separated by column chromatography with a mixture of petroleum ether / dichloromethane (1:1 by volume), and finally 0.144 g of a dark green solid was obtained, with a yield of 15%.
[0102] The reaction formula of preparing above-mentioned compound Q2 is as follows:
[0103]
[0104] see Figure 4 , is the normalized UV-Vis spectrum absorption figure of compound Q2 in chloroform solution and...
Embodiment 3
[0108] This example discloses the synthesis of compound Q3, the specific synthesis steps are as follows:
[0109] Under nitrogen, the compound ABr (1.896g, 4.00mmol), tetrakis (triphenylphosphine) palladium (46mg, 0.040mmol) was dissolved in 20mL of toluene, and then containing 9,9-dioctyl-2,7 - A 20 mL toluene solution of bis{5-(tributyltin-yl)-thiophen-2-yl}fluorene (1.812 g, 1.60 mmol) was injected into the reaction system, and refluxed at 110° C. for 48 h. The end of the reaction was detected by TLC, and the solvent in the reaction solution was removed. The crude product was separated by column chromatography with a mixture of petroleum ether / dichloromethane (1:2 by volume), and finally 0.150 g of a dark green solid was obtained, with a yield of 7%.
[0110] The reaction formula of preparing above-mentioned compound Q3 is as follows:
[0111]
[0112] see Figure 7 , is the normalized UV-Vis spectrum absorption figure of compound Q3 in chloroform solution and film-for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com