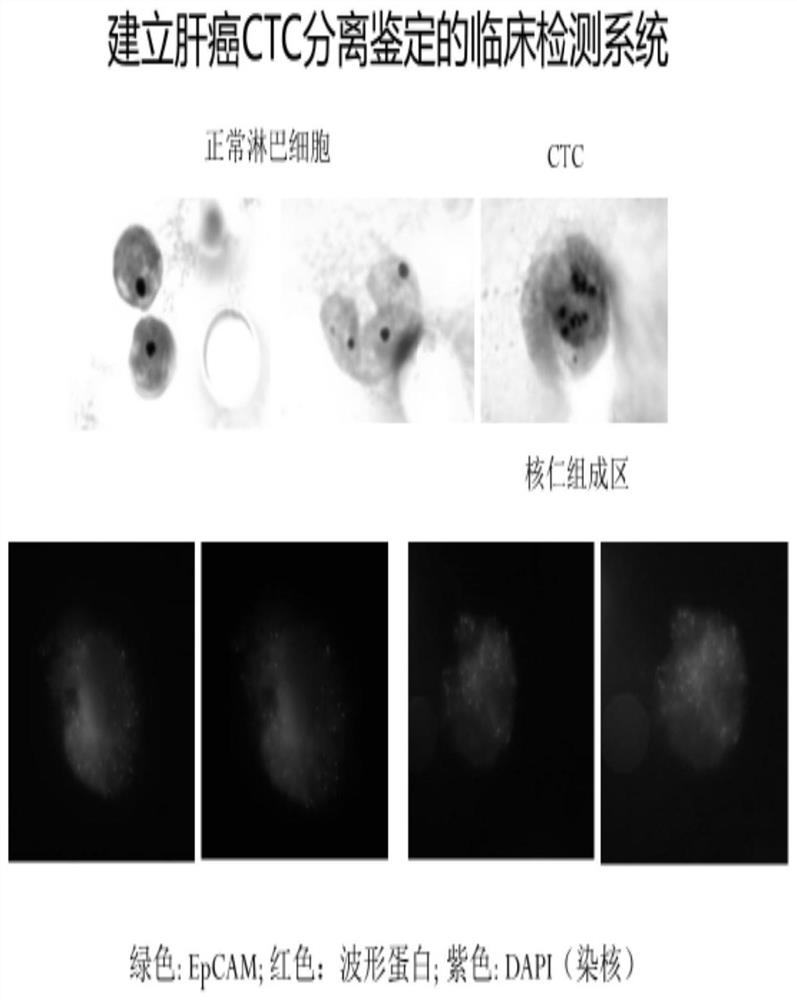
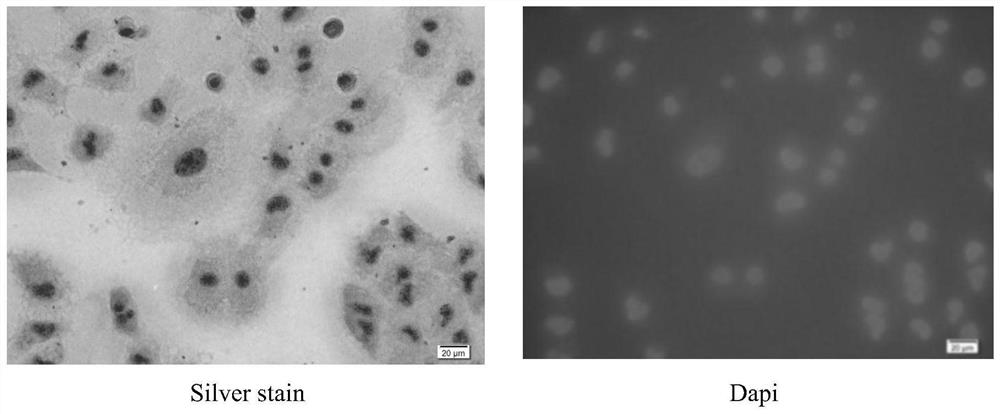
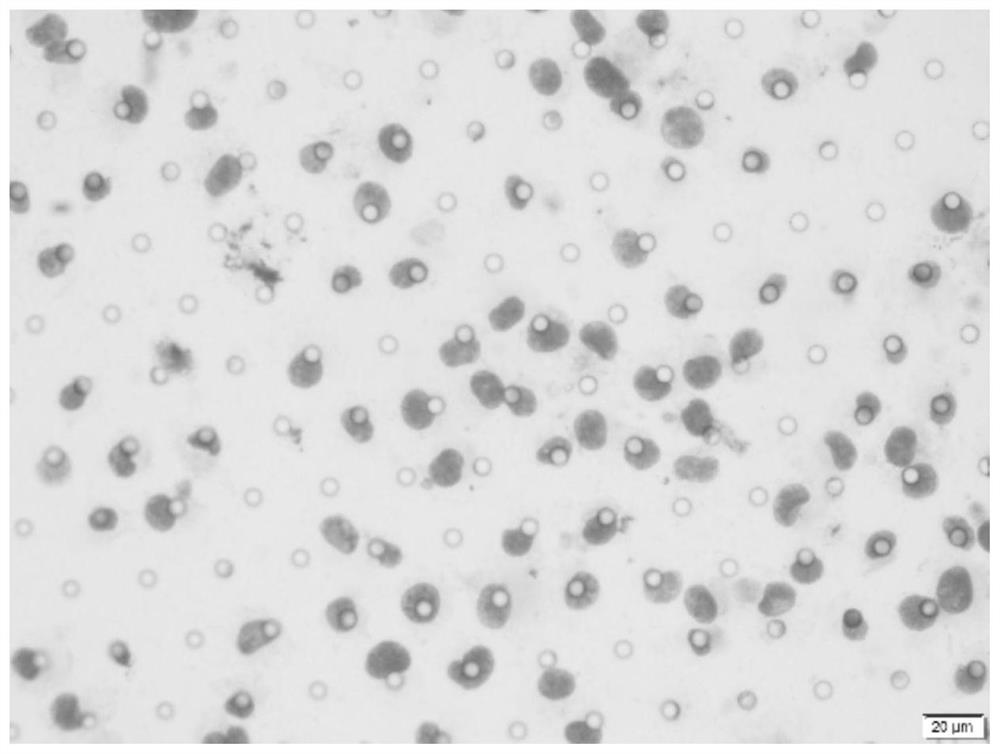
Novel circulating tumor cell identification technology
A new type of tumor cell technology, applied in the field of medical diagnosis, can solve the problems of false positives, imperfect detection methods, and difficulty in accurate quantification by fluorescence microscopy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Collection, transportation and storage of peripheral blood, sample pretreatment:
[0052] According to the standard sampling procedure, take 3-5 ml with BD EDTA anticoagulant tube, press the angle of 90 degrees, shake gently up and down 8 times, and transport the blood sample with a low-temperature ice pack (8°C). If left at room temperature, it needs to be used for experimental operations within 2 hours, or it can be stored in a 4-degree refrigerator for about a day.
[0053] (2) Enrichment and separation of CTCs: Enrichment and separation were carried out with a CTC capture device, and separated on a microporous membrane.
[0054] (3) Cell immobilization on microporous membrane: cell morphology identification and immunofluorescence detection of CTC;
[0055] CTCs need to be fixed after being enriched on the membrane. The fixative: 90% alcohol, 5% of 1x PBS and 5% acetic acid are prepared immediately before use. Add 1ml of fixative to the cell strainer, fix for at...
Embodiment 2
[0065] (1) Collection, transportation and storage of peripheral blood, sample pretreatment:
[0066] According to the standard sampling procedure, take 3-10 ml of BD EDTA anticoagulant tube, press the angle of 90 degrees, shake gently up and down 8 times, and transport the blood sample with a low-temperature ice pack (4°C). If left at room temperature, it needs to be used for experimental operations within 2 hours, or it can be stored in a 4-degree refrigerator for about one day.
[0067] (2) Enrichment and separation of CTCs: Enrichment and separation were carried out with a CTC capture device, and separated on a microporous membrane.
[0068] (3) Cell immobilization on microporous membrane: cell morphology identification and immunofluorescence detection of CTC;
[0069] CTCs need to be fixed after being enriched on the membrane. The fixation solution: 90% alcohol, 5% of 1x PBS and 5% acetic acid, prepared immediately before use, can be added to the cell strainer with 1ml fi...
Embodiment 3
[0079] (1) Collection, transportation and storage of peripheral blood, sample pretreatment:
[0080] According to the standard sampling procedure, take 3-5 ml with BD EDTA anticoagulant tube, press the angle of 90 degrees, shake gently up and down 8 times, and transport the blood sample with a low-temperature ice pack (6°C). If left at room temperature, it needs to be used for experimental operations within 2 hours, or it can be stored in a 4-degree refrigerator for about one day.
[0081] (2) Enrichment and separation of CTCs: Enrichment and separation were carried out with a CTC capture device, and separated on a microporous membrane.
[0082] (3) Cell immobilization on microporous membrane: cell morphology identification and immunofluorescence detection of CTC;
[0083] CTCs need to be fixed after being enriched on the membrane. The fixation solution: 90% alcohol, 5% of 1x PBS and 5% acetic acid, prepared immediately before use, can be added to the cell strainer with 1ml f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Pore diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com