Valproic acid drug concentration detection kit and application thereof
A technology for detecting the concentration of kits and drugs, which is applied in the field of medical testing and can solve problems such as high requirements for storage and transportation conditions, large sample volume, and endogenous interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
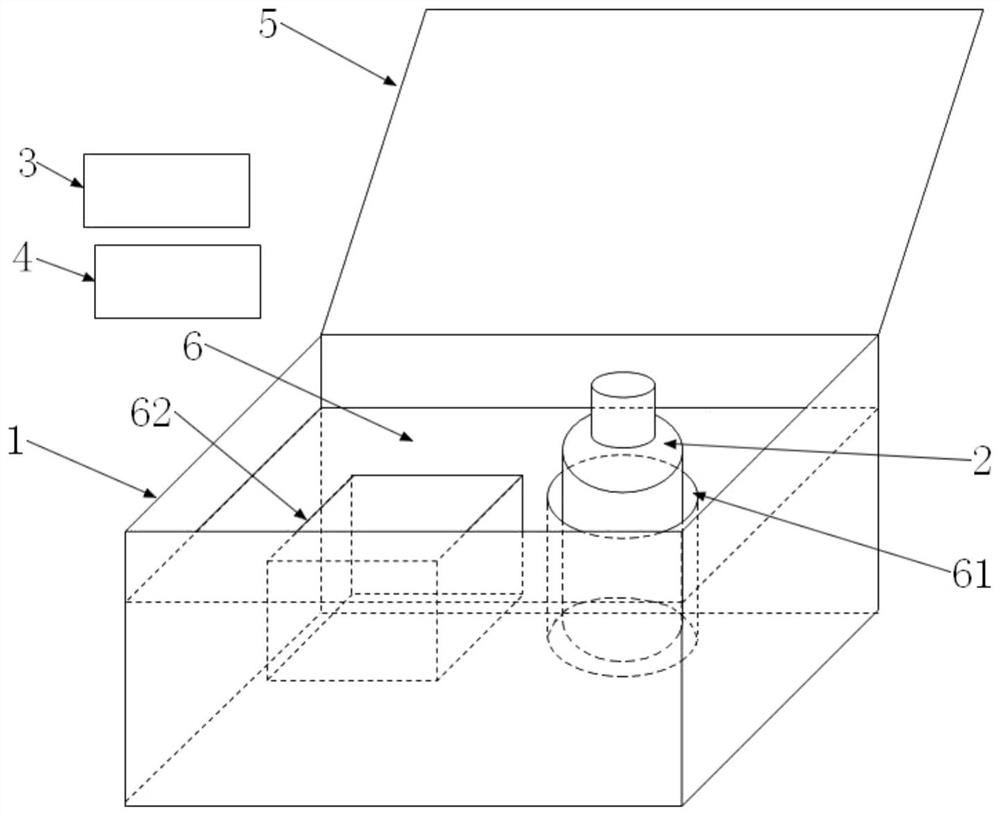
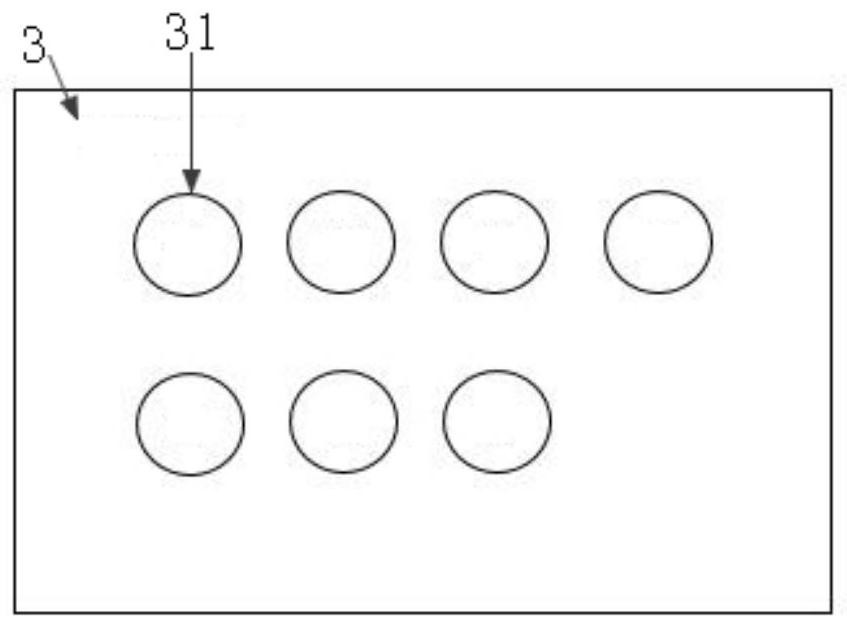
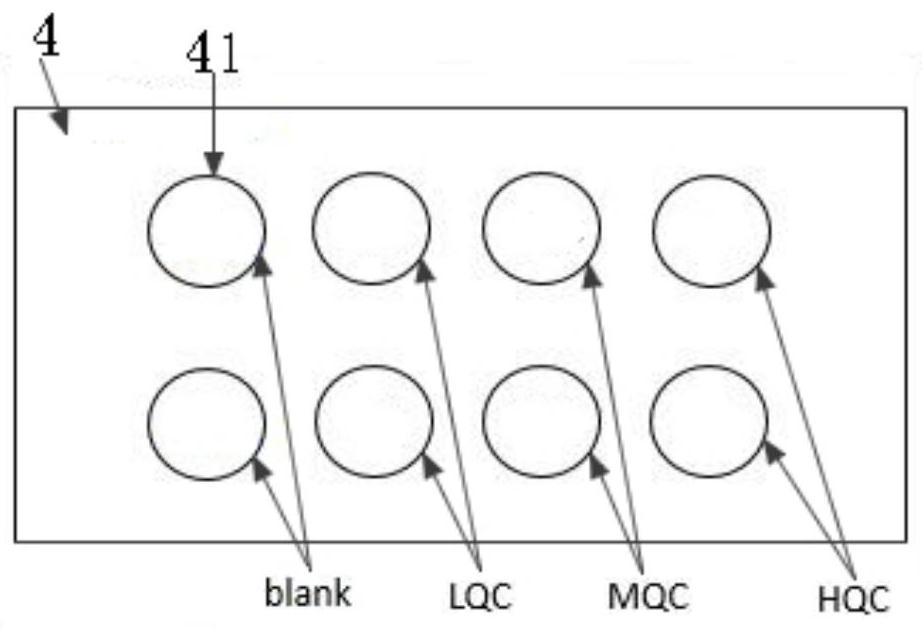
[0079] Such as figure 1 Shown is a schematic diagram of the three-dimensional structure of a specific embodiment of the kit of the present invention. The valproate concentration detection kit includes: a box body 1, a first container 2, a calibrator card 3 and a quality control card 4. Wherein, the first container 2 , the calibration product card 3 and the quality control product card 4 are placed in the box body 1 .
[0080] figure 1 The reagent box shown in also includes a box cover 5, and the box cover 5 cooperates with the box body 1 to realize the function of closing or opening the reagent box. figure 1 In the above, the box cover 5 is connected with the box body 1 and is integrated; in other embodiments, the box cover 5 and the box body 1 can also be independent of each other, which is a separate type. When the box cover 5 cooperates with the box body 1 to close, the reagent box can be kept in a sealed state.
[0081] figure 1 The kit shown in , also includes a fixa...
Embodiment 2
[0096] Example 2 The preparation process of the kit
[0097] (1) Preparation of standard solution
[0098] Accurately weigh 30 mg of valproic acid compound, place it in a 10 mL volumetric flask, dissolve in pure methanol, and set the volume to 10 mL to obtain a stock solution with a valproic acid concentration of 3 mg / mL.
[0099] (2) Preparation of sample extract
[0100] Take 20 μL of valproic acid internal standard (VPA-D6) stock solution (1mg / mL), dilute to 40mL with 80% methanol, and prepare the sample extraction solution containing internal standard (VPA-D6) concentration of 0.5 μg / mL 80 % methanol solution.
[0101] (3) Calibration sample card and quality control sample card preparation
[0102] 1) After mixing the freshly collected blood from 6 rabbits, measure the mixed rabbit hematocrit (HCT) according to the laboratory "Hematocrit Measurement and Adjustment Standard Operating Procedures" (SD1-S-M3008), and finally adjust the mixed blood The HCT value of rabbit b...
Embodiment 3
[0130] Embodiment three: use the test kit of the present invention to carry out the feasibility study of valproic acid method (linear experiment, specificity experiment)
[0131] 1. Sample preparation
[0132] Sample preparation was carried out according to the methods of "(1) preparation of standard solution", "(2) preparation of sample extract", "(3) preparation of calibration sample card and quality control sample card" in Example 2.
[0133] The blood sample is directly collected on a specific filter paper to form a dried blood spot sample that meets the requirements, and a small blood spot disc of an appropriate size is punched from the center of the dried blood spot with a puncher and placed in a 96-well plate; Valproic acid-D6 internal standard solution with a concentration of 0.5 μg / mL was vortexed for 10 minutes to complete the extraction and precipitation process; solid impurities were removed by centrifugation, and an appropriate amount of supernatant was added to a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com