High-sensitivity fluorescent probe for detecting Hg<2+>, preparation method and application thereof
A fluorescent probe, high-sensitivity technology, applied in the field of high-sensitivity fluorescent probes for detecting Hg2+ and their preparation, can solve the problems of low sensitivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
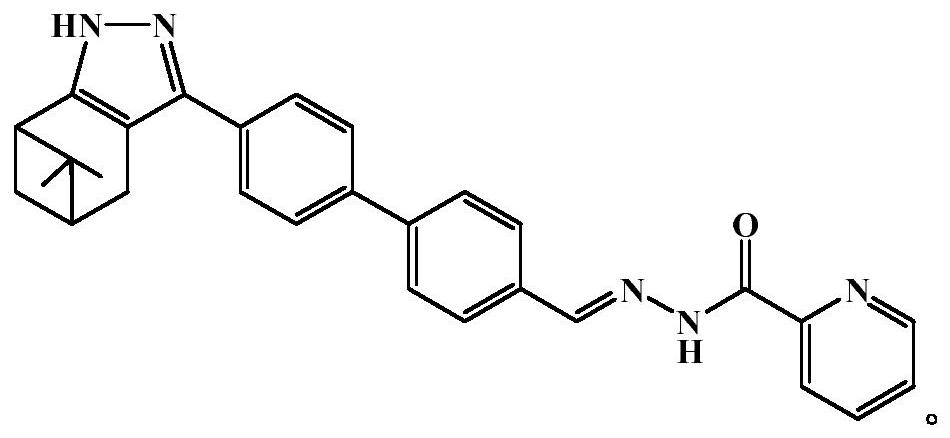
[0035] N'-(4'-(6,6-dimethyl-4,5,6,7-tetrahydro-1H-5,7-methanoindazol-3-yl)-[1,1' Preparation of -biphenyl]-4-methylene)pyridine-2-carbohydrazide
[0036] 1) Preparation of 3-(4-bromobenzoyl)nopinone:
[0037] Add 3mmol nopinone, 3.6mmol methyl 4-bromobenzoate, 9mmol sodium hydride and 60mL ethylene glycol dimethyl ether into a three-necked flask in turn, heat and reflux reaction under nitrogen protection, and react for about 8 hours until the conversion rate of nopinone Up to 95% or more (TLC tracking detection). After the reaction solution was cooled, 30 mL of deionized water was added to quench the reaction, and then extracted 3 times with 0.3 L of ethyl acetate, and the combined organic phase was washed several times with saturated brine until neutral, dried over anhydrous sodium sulfate, and filtered , the solid product obtained after concentration, and then washed with a small amount of ethyl acetate to obtain 3-(4-bromobenzoyl)nopinone, with a yield of 62.1% and a puri...
Embodiment 2
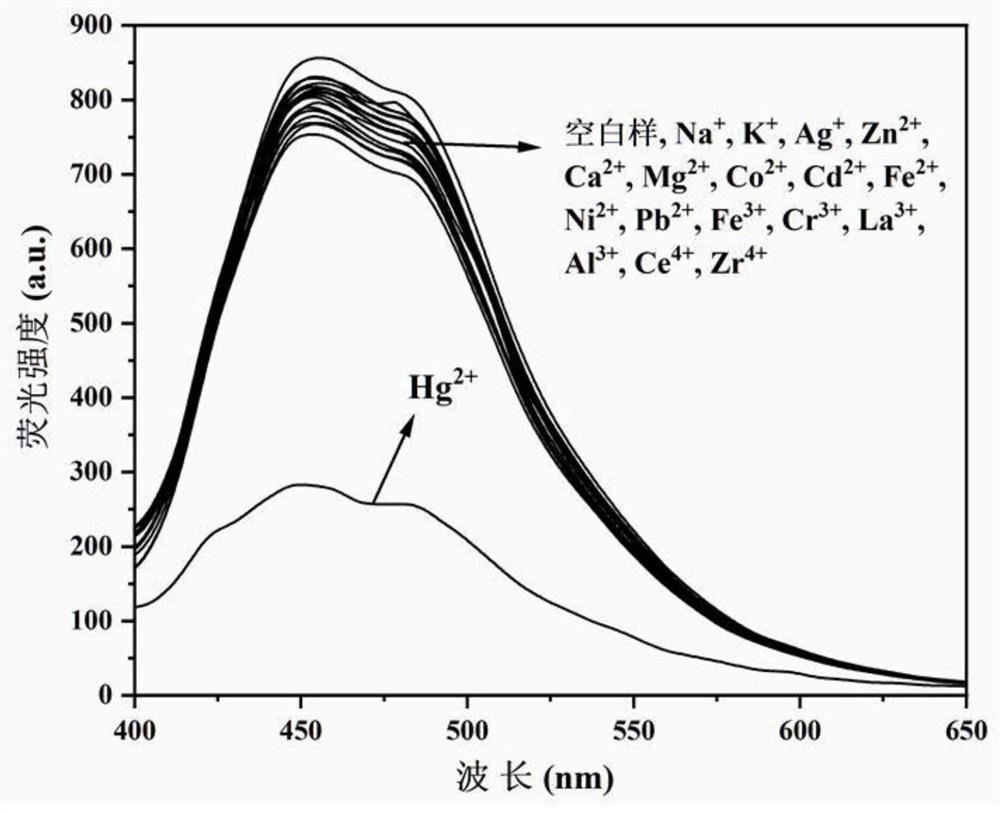
[0045] N'-(4'-(6,6-dimethyl-4,5,6,7-tetrahydro-1H-5,7-methanoindazol-3-yl)-[1,1 '-Biphenyl]-4-methylene)pyridine-2-carboxylhydrazide was dissolved in DMF to prepare a solution with a concentration of 1 mM, and diluted to 10 μM with PBS buffer solution (pH=7.4, 10 mM) for use. Different metal ions were dissolved in deionized water and diluted to 100 μM for use, and different metal ion pairs N'-(4'-(6,6-dimethyl-4,5,6, Fluorescence spectrum of 7-tetrahydro-1H-5,7-methanoindazol-3-yl)-[1,1′-biphenyl]-4-methylene)pyridine-2-carboxhydrazide, Such as figure 1 shown. The results showed that, compared to other metal ions, only Hg 2+ It can cause obvious changes in the fluorescence spectrum of the compound, indicating that the compound can specifically recognize Hg 2+ .
Embodiment 3
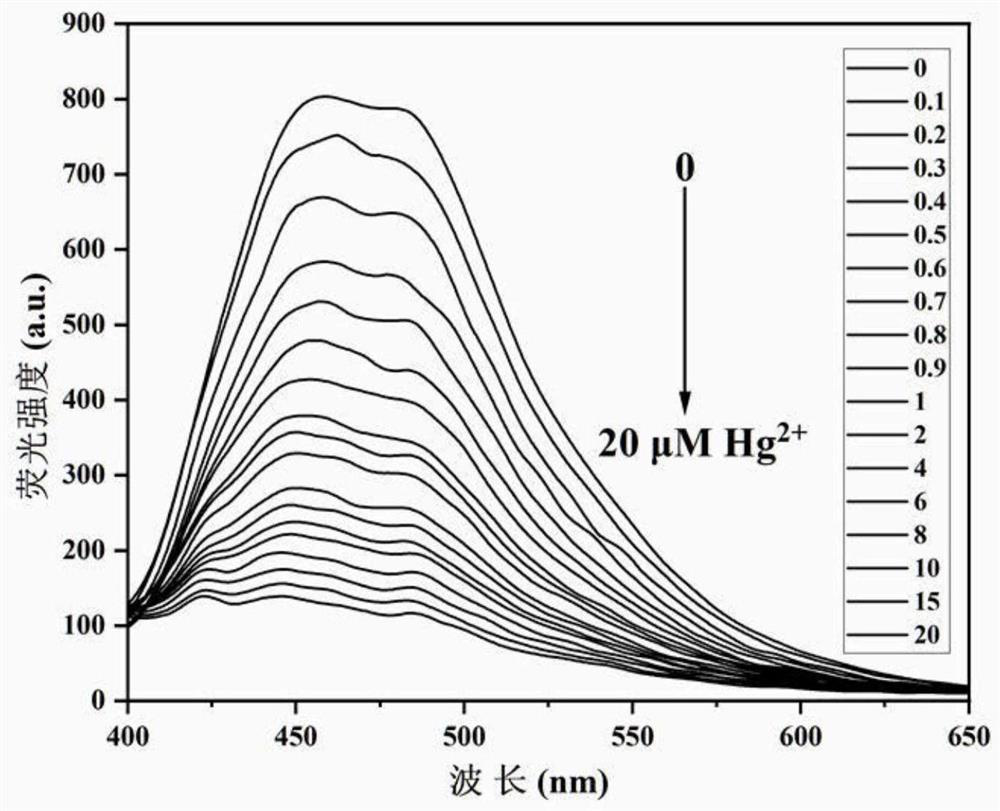
[0047] N'-(4'-(6,6-dimethyl-4,5,6,7-tetrahydro-1H-5,7-methanoindazol-3-yl)-[1,1 '-Biphenyl]-4-methylene)pyridine-2-carboxylhydrazide was dissolved in DMF to prepare a solution with a concentration of 1 mM, and then diluted to 10 μM with PBS buffer solution (pH=7-4, 10 mM) for use. Also Hg 2+ Dissolve in PBS buffer solution to prepare solutions with concentrations of 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 2, 4, 6, 8, 10, 15, 20 μM. The different concentrations of Hg were measured by fluorescence spectrometry 2+On N'-(4'-(6,6-dimethyl-4,5,6,7-tetrahydro-1H-5,7-methanoindazol-3-yl)-[1,1 The fluorescence spectrum of '-biphenyl]-4-methylene)pyridine-2-carbohydrazide, such as figure 2 shown. The results showed that the fluorescence intensity of the compound at around 460nm was obviously quenched gradually, indicating that the compound could be used as a tool for detecting Hg 2+ fluorescent probes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com