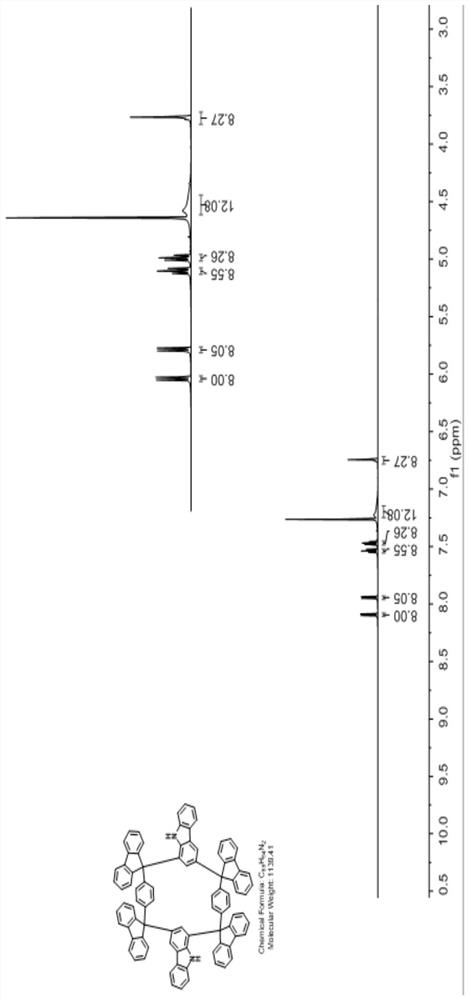
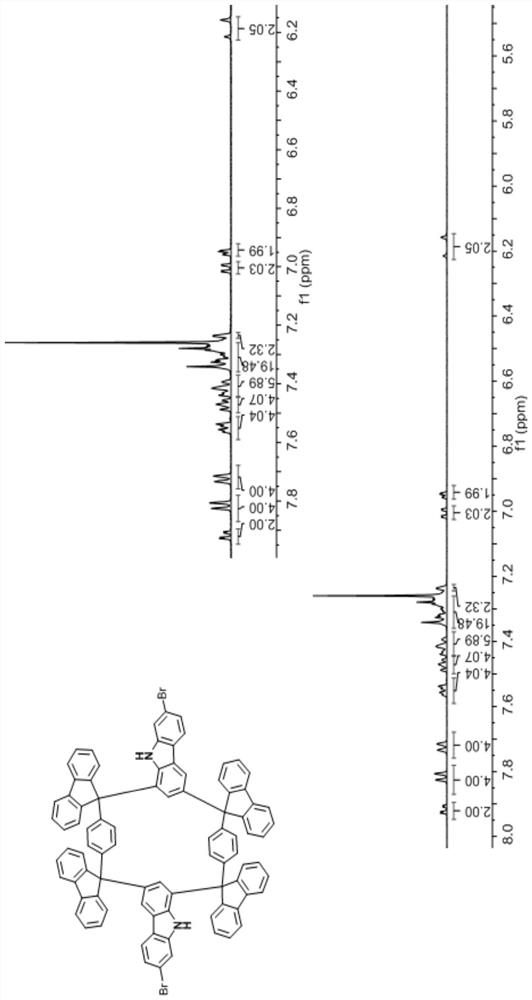
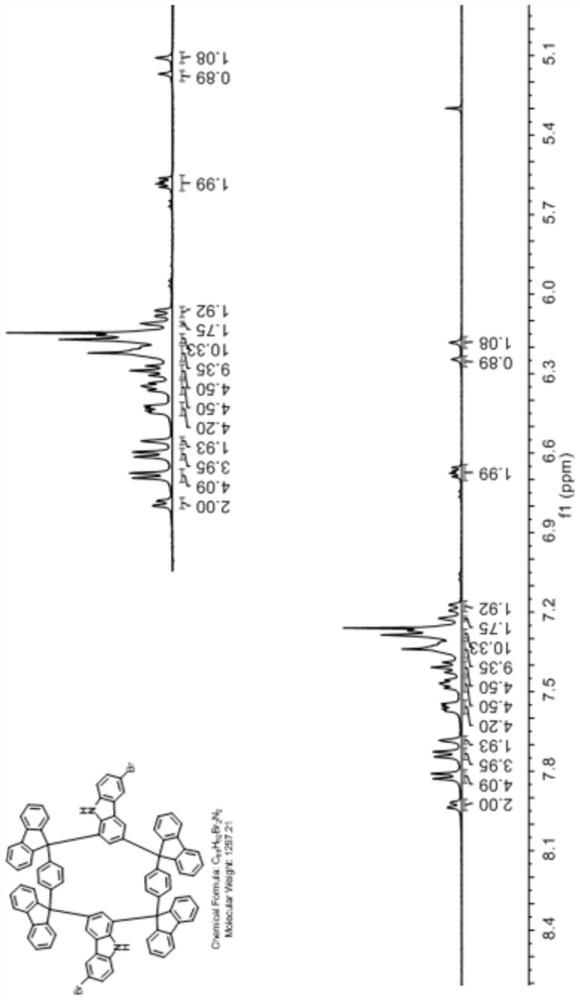
Carbazole 1, 3-site Friedel-Crafts ring-closing cyclic compound and preparation method thereof
A technology for cyclic compounds and compound structures, applied in organic chemistry and other directions, can solve the problems of difficult separation and low synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] A kind of preparation method of the cyclic compound of carbazole 1,3 Friedel-Crafts ring closure, specifically comprises the following steps:
[0041] The first step: synthesize the Grignard reagent from p-bromobenzene or its derivatives, and then react the prepared Grignard reagent with fluorenone or its derivatives to obtain a type I di-tertiary alcohol synthon;
[0042] The second step: the obtained type I di-tertiary alcohol synthon is reacted with carbazole or its derivatives at room temperature with an acid as a catalyst in a dry organic solvent to obtain the carbazole 1,3 A cyclic compound with a Friedel-Crafts ring.
[0043]
Embodiment 1
[0045] Embodiment 1: the preparation of DBDFOH-I type tertiary alcohol synthon 1
[0046] Dry all the glass instruments to be used in advance (magnets, long needles, two two-necked flasks, and two spherical condenser tubes); steam THF in advance (during the distillation of tetrahydrofuran, a small amount of benzophenone needs to be added to color, when there is no water in the flask, the bottle turns dark blue, at this time tetrahydrofuran can be collected for experiment); then the glass instrument is assembled, magnesium grains (3.06g, 0.127mol) and a grain of iodine (2.54mg, 0.02 mmol) was added (vacuum grease, sealing tape) to one of the instruments, vacuumed three times, and protected by a nitrogen balloon. Dissolve the steamed THF (60 mL) in 1,4-dibromobenzene (10 g, 0.042 mol) that has been evacuated and supplemented with nitrogen, dissolve it, inject it into the reactor with a syringe, and blow hot air to initiate the reaction. Then slowly add the drug (the remaining d...
Embodiment 2
[0047] Embodiment 2: Preparation of LBDFOH-I type tertiary alcohol synthon 2
[0048] Dry all the glass instruments to be used in advance (magnets, long needles, two two-necked flasks, and two spherical condenser tubes); steam THF in advance (during the distillation of tetrahydrofuran, a small amount of benzophenone needs to be added to color, when there is no water in the flask, the bottle turns dark blue, at this time tetrahydrofuran can be collected for experiment); then the glass instrument is assembled, magnesium grains (2.34g, 0.096mol) and a grain of iodine (2.54mg, 0.02 mmol) was added (vacuum grease, sealing tape) to one of the instruments, vacuumed three times, and protected by a nitrogen balloon. Dissolve the evaporated THF (90mL) in 4,4'-dibromobiphenyl (10g, 0.032mol) that has been evacuated and supplemented with nitrogen, dissolve it, inject it into the reactor with a syringe, and blow hot air to initiate the reaction. Then slowly add the drug (the remaining dru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com