Isoniazide derivative, homogeneous enzyme immunoassay reagent and preparation method
A homogeneous enzyme immunoassay and detection reagent technology, which is applied in the direction of immunoglobulin, chemical instruments and methods, measuring devices, etc., can solve complex operations, cannot meet the requirements of high-throughput and rapid clinical therapeutic drug monitoring, and take a long time And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
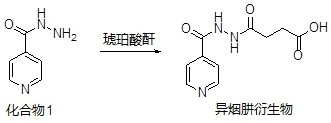
[0071] Embodiment 1: the synthesis of isoniazid derivative
[0072] The synthetic route of isoniazid derivative is as follows:
[0073] .
[0074] The preparation method of isoniazid derivative, concrete steps are as follows:
[0075] Dissolve 2 g of compound 1 in 100 ml of toluene, then add 1.4 g of succinic anhydride to prepare a reaction mixture solution, reflux and stir the reaction mixture overnight, then concentrate the reaction mixture, and finally use flash chromatography to obtain 0.9 g of solid isofume Hydrazine derivatives.
[0076] passed 1 H NMR (Varian mercury plus 400 MHz) spectral scanning analysis (TMS as an internal standard) and LC-MS (Agilent 1200A) were used to identify the structure of the derivative. The results showed that the isoniazid derivative was represented by the formula (Ⅴ) The isoniazid derivatives shown.
[0077] Formula (Ⅴ).
Embodiment 2
[0078] Example 2: Preparation of Isoniazid Immunogen
[0079] The preparation method of isoniazid immunogen comprises the following steps:
[0080] (A1) Preparation of carrier solution: Dissolve carrier protein in 0.2M potassium phosphate buffer (pH=8.5), the final concentration of carrier protein is 4mg / mL, to obtain carrier solution;
[0081] (A2) Preparation of isoniazid derivative solution: 200 mg of the above-mentioned isoniazid derivative, 4 mL of dimethylformamide, 4 mL of ethanol, 5 mL of potassium phosphate buffer (10 mM, pH=5.0), 200 mg of 1-butane Base-3-(-3-dimethylaminopropyl) carbodiimide and 50 mg N-hydroxysulfosuccinimide were mixed, stirred and dissolved for 90 minutes to obtain a solution of isoniazid derivatives;
[0082] (A3) Synthesis of isoniazid immunogen: add the isoniazid derivative solution obtained in step (A2) to the carrier solution obtained in step (A1), stir overnight at 4°C, and purify by dialysis to obtain isoniazid Niacinamide immunogen.
Embodiment 3
[0083] Example 3: Preparation of Isoniazid Immunogen
[0084] The preparation method of isoniazid immunogen comprises the following steps:
[0085] (A1) Preparation of carrier solution: Dissolve carrier protein in 0.2M potassium phosphate buffer (pH=8.5), the final concentration of carrier protein is 3mg / mL to obtain carrier solution;
[0086] (A2) Preparation of isoniazid derivative solution: 100 mg of the above-mentioned isoniazid derivative, 2 mL of dimethylformamide, 2 mL of ethanol, 3 mL of potassium phosphate buffer (10 mM, pH=5.0), 100 mg of 1-butane Base-3-(-3-dimethylaminopropyl)carbodiimide and 20mg N-hydroxysulfosuccinimide were mixed, stirred and dissolved for 30 minutes to obtain a solution of isoniazid derivatives;
[0087] (A3) Synthesis of isoniazid immunogen: add the isoniazid derivative solution obtained in step (A2) to the carrier solution obtained in step (A1), stir overnight at 0°C, and purify by dialysis to obtain isoniazid Niacinamide immunogen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com