Preparation method of platelet aggregation inhibitor ticagrelor
A technology of platelet aggregation and ticagrelor, applied in the direction of organic chemistry, can solve the problems of no conformational change and signal transmission, and achieve the effect of less impurities, less by-products, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
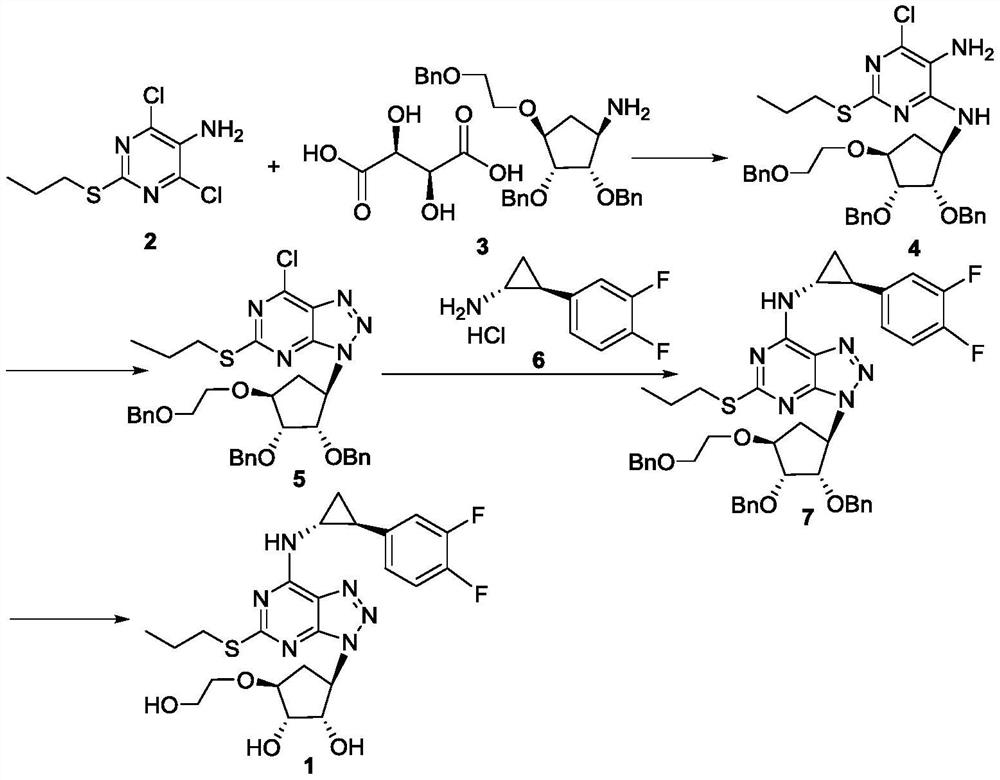
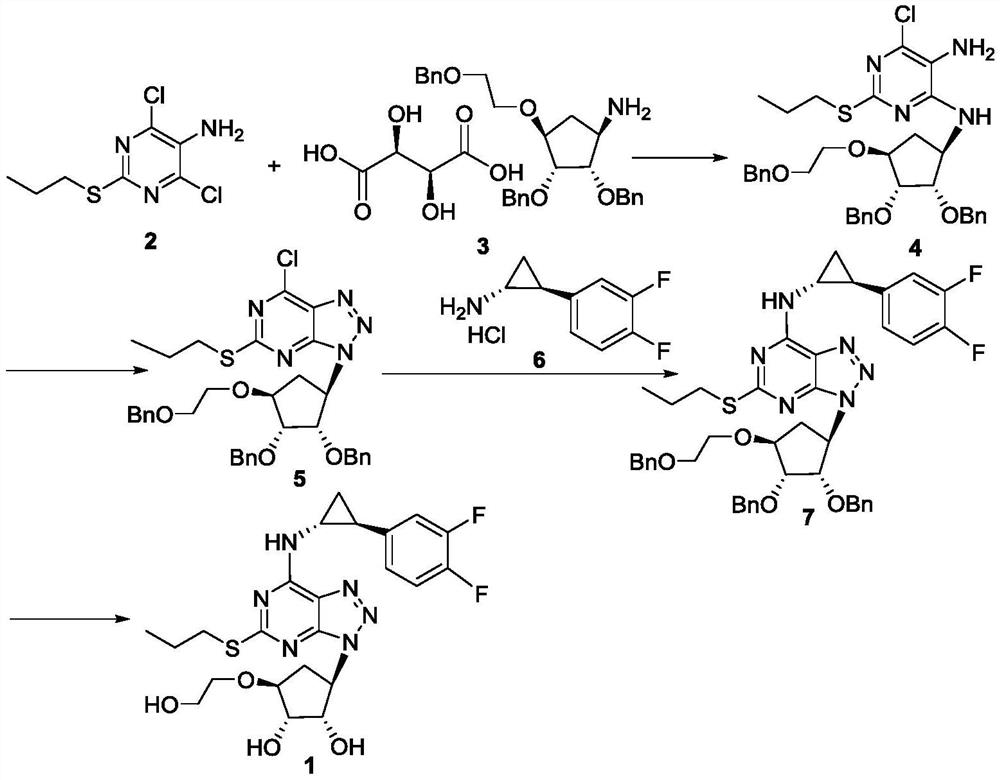
[0022] Add compound 2 (1.00g, 4.20mmol), compound 3 (2.76g, 4.62mmol) and 30mL isopropanol successively to a 100mL reaction flask, add triethylamine (1.61g, 15.94mmol) under stirring, and heat up to 80°C , keep the reaction for 20 hours, cool to room temperature, transfer the reaction solution to a 100mL reaction bottle, add 30mL water and 10mL ethyl acetate, stir and leave to separate layers, extract the water phase with 10mL ethyl acetate twice, combine the organic phase and wash with water , Wash with saturated saline. Dry over anhydrous sodium sulfate and filter. After concentration and spin-drying, 2.342 g of a dark red solid (compound 4) was obtained, with a yield of 86%. 1 H-NMR (CDCl 3 , 400MHz) δ: 0.94(t, J=7.2Hz, 3H), 1.60-1.66(m, 2H), 2.08-2.15(m, 1H), 2.92-3.01(m, 2H), 3.01-3.04(m, 2H), 3.13-3.15(m,1H), 3.46(m,1H), 3.68-3.71(m,2H), 3.77-3.80(m,2H), 4.31-4.32(m,1H), 4.65(d, 6H), 5.68(s,2H), 7.29-7.32(m,15H), 7.62(s,1H); ESI-MS m / z:C 35 h 41 ClN 4 o 4 S[M+H] ...
Embodiment 2
[0028] Add compound 2 (1.00kg, 4.20mol), compound 3 (3.01kg, 5.04mol) and 30L isopropanol successively in 50L enamel reaction kettle, add triethylamine (1.70kg, 16.80mol) under stirring, heat up to 80 ℃, heat preservation reaction for 30 hours, cooled to room temperature, transferred the reaction solution to a 100L reaction kettle, added 30L water and 10L ethyl acetate, stirred, left to stand for liquid separation, extracted the water phase twice with 10L ethyl acetate, and combined The organic phase was washed twice with 10 L of water and washed with 10 L of saturated brine. Dry over anhydrous sodium sulfate and filter. After concentration and spin-drying, 2.42 kg brick red solid (compound 4) was obtained, with a yield of 89%.
[0029] 50L of acetonitrile, compound 4 (2.42kg, 3.73mol) and isoamyl nitrite (0.55kg, 4.69mol) were sequentially added to a 100L reactor, stirred, and then heated to 75°C for 12 hours under reflux. After the reaction was detected by TLC, it was conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com