Ion-modified gallium protoporphyrin compound as well as preparation method and application thereof
An ion modification and compound technology, which is applied in the field of ion modified protoporphyrin gallium compounds and their preparation, can solve problems such as abuse and multidrug-resistant bacteria, and achieves the promotion of killing effect, overcoming bacterial resistance, high reactive oxygen species and the like. volume effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
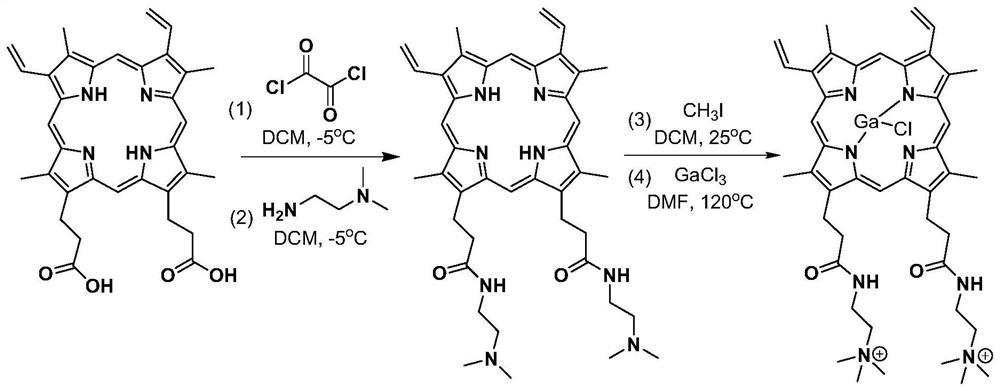
[0039] Example 1: Gallium (III) dimethyl-8,13-divinyl-3,7,12,17-tetramethyl-21H, 23H-porphyrin-2,18-bis[-N,N, Preparation of N-trimethyl-2-(propionyl ammonium)](CMP-Ga)
[0040] Synthetic route see figure 1 .
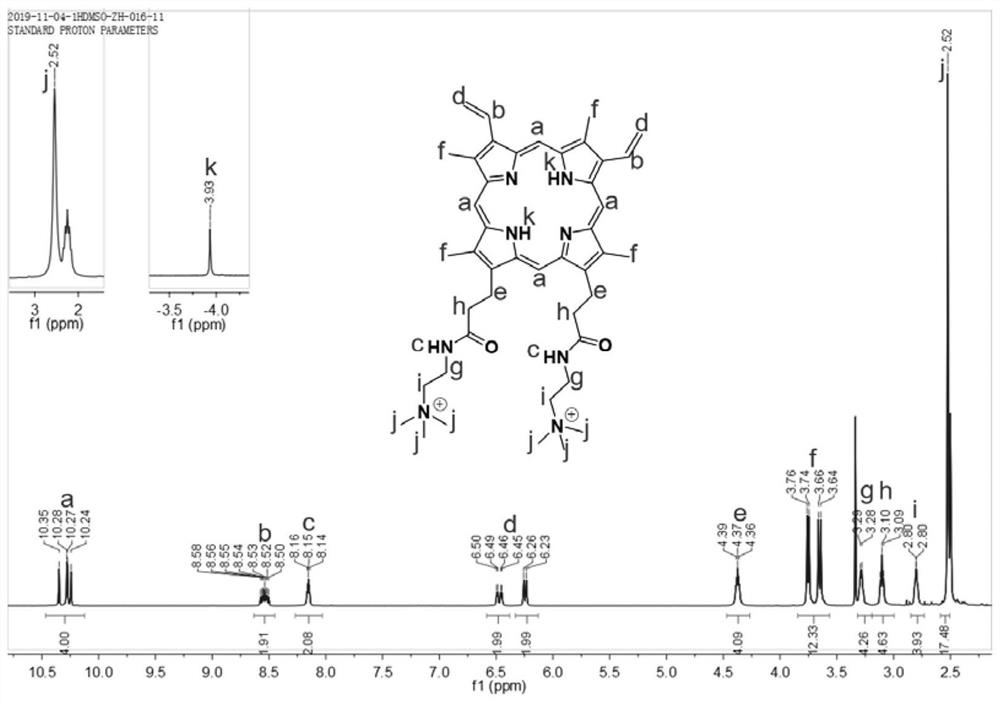
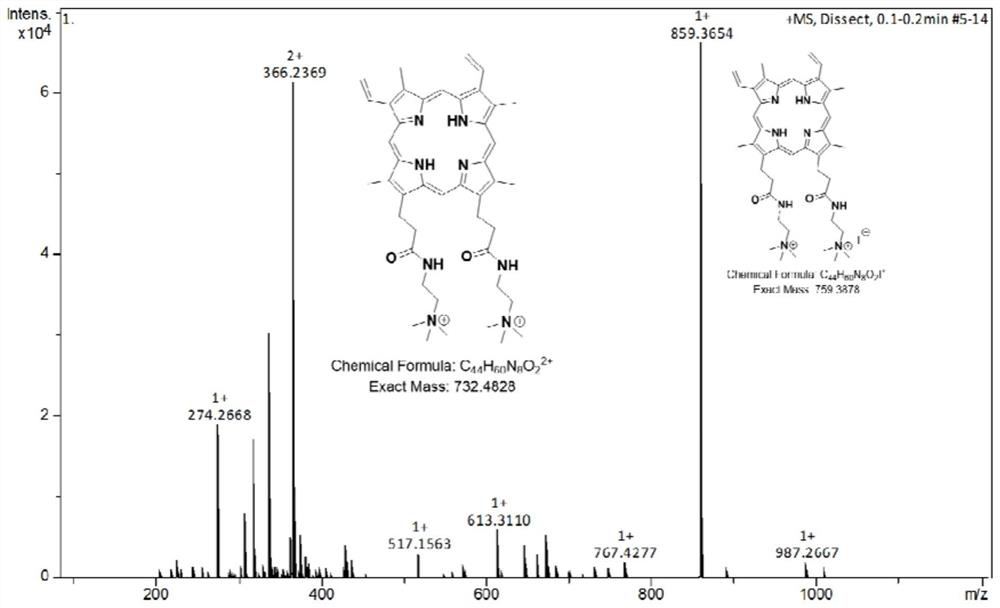
[0041] Weigh 100.0 mg of protoporphyrin into a reaction flask, add 200 mL of dichloromethane, stir to dissolve, cool down to -5°C, slowly add 22.6 mg of oxalyl chloride dropwise, stir for 1 hour, and rotate to evaporate the solvent and excess oxalyl chloride ;Add 200mL of dichloromethane again, stir to dissolve, cool down to -5°C, slowly add 15.7mg of N,N-dimethylethylenediamine dropwise, stir for 6h, rotary evaporate the solvent, add 400mL of deionized water, Stir for 6 hours, filter, rinse with deionized water, and vacuum-dry the filter cake at 60°C; Rinse with methane, and vacuum dry the filter cake at 40°C to obtain cation-modified protoporphyrin (CMP, see Figure 2-3 ); the cation-modified protoporphyrin was dissolved in 200mL of ultra-dry N,N-dimethylformamide...
Embodiment 2
[0042] Example 2: Gallium (III) dimethyl-8,13-divinyl-3,7,12,17-tetramethyl-21H, 23H-porphyrin-2,18-bis[-N-(carboxy Methyl)-N, N-dimethyl-2-(propionyl ammonium)], the preparation of internal salt (ZMP-Ga): the synthetic route sees Figure 5 .
[0043] Weigh 100mg of protoporphyrin into the reaction flask, add 200mL of tetrahydrofuran, stir to dissolve, cool down to 5°C, slowly add 45.1mg of oxalyl chloride dropwise, stir for 6h, rotary evaporate the solvent and excess oxalyl chloride; add 200mL again THF, stir to dissolve, cool down to 5°C, slowly add 23.5mL of N,N-dimethylethylenediamine dropwise, stir for 12h, rotary evaporate the solvent, add 400mL of deionized water, stir for 6h, filter, use Rinse with deionized water, dry the filter cake under vacuum at 60°C; dissolve the obtained filter cake with 200 mL of tetrahydrofuran, slowly add 37.0 mg of bromoacetic acid dropwise at room temperature, react at 60°C for 12 hours, filter, rinse with tetrahydrofuran, and dry the filt...
Embodiment 3
[0044] Example 3: Gallium(III) dimethyl-8,13-diethyl-3,7,12,17-tetramethyl-21H,23H-porphyrin-2,18-bis[-N-(sulfonic Propyl)-N, N-dimethyl-2-(butyryl ammonium)], the preparation of inner salt (SMP-Ga): see the synthetic route Figure 9 .
[0045] Weigh 100mg of Meso-protoporphyrin into a reaction flask, add 200mL of acetone, stir to dissolve, cool down to 0°C, slowly add 33.6mg of oxalyl chloride dropwise, stir for 3 hours, and rotate to evaporate the solvent and excess oxalyl chloride; Add 200mL of acetone, stir to dissolve, cool down to 0°C, slowly add 21.6mL of N, N-dimethylpropylenediamine dropwise, stir for 8h, rotate to evaporate the solvent, add 400mL of deionized water, stir for 6h, filter, Rinse with deionized water, vacuum-dry the filter cake at 60°C; dissolve the obtained filter cake with 200mL of acetone, slowly add 42.8mg of 3-bromo-1-propanesulfonic acid dropwise at room temperature, react at 40°C for 8h, filter, and rinse with acetone After washing, the filter c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com