Construction and application of threonine dehydratase mutant
A technology of threonine dehydratase and mutants, applied in the direction of application, enzyme, lyase, etc., can solve the problems of backward extraction technology of fermentation products, backward fermentation technology, unable to solve the problem of feedback inhibition of key enzymes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1, the acquisition of threonine dehydratase mutant
[0015] 1. Threonine dehydratase expression plasmid and realization of site-directed mutagenesis
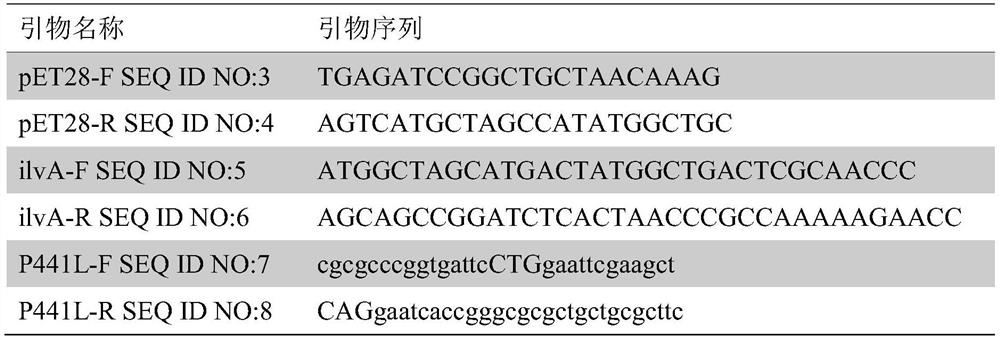
[0016] The threonine dehydratase in Escherichia coli is obtained from the genome of Escherichia coli from the ilvA gene by PCR. Plasmid pET28a was also amplified by PCR to obtain a common repeat sequence, and ligated by In Fusion enzyme to obtain plasmid pET28a-ilvA. The primers used in the experiment are listed in Table 1.
[0017] Gene site-directed mutagenesis using the Stratagene series The XL-II site-directed mutagenesis kit is realized by introducing the mutation site P441L into the plasmid pET28a-ilvA by PCR with primers P441L-F / P441L-R (see Table 1), that is, replacing the proline at position 441 of ilvA with leucine. The obtained plasmid was recovered by PCR product, and after removing the enzyme in the PCR system and the salt ion in the buffer system, it was digested with Dpn1 for 1 hour to remove...
Embodiment 2
[0020] Embodiment 2, in vitro effect detection of ilvA mutant
[0021] 1. Protein expression and purification
[0022] First pick BL21 (DE3) (pET28a-ilvA) (overexpression of ilvA wild-type gene) from the plate, single colonies of BL21 (DE3) (pET28a-ilvA) (overexpression of ilvA-P441L mutant gene) were inoculated in 5ml containing 50μg / ml kanapenicillin LB medium, cultured at 37 degrees and 200 rpm for 5 hours until the OD600 was about 1.0. Take 2ml of the primary seed solution with an OD of about 1.0, transfer it to 100ml of fresh LB medium containing 100μg / ml kanapenicillin, and cultivate overnight at 20°C. After the bacteria were collected, the bacteria were disrupted by ultrasonic for 10 minutes, and the ultrasound was stopped for 1 second for 3 seconds, and the protein was purified with a nickel column. The protein was quantified by BCA (Bicinchoninic Acid) method, and the protein purity was determined by SDS-PAGE. The results showed that the protein purity was greater...
Embodiment 3
[0026] Embodiment 3: the application of mutant
[0027] 1. Fermentation method
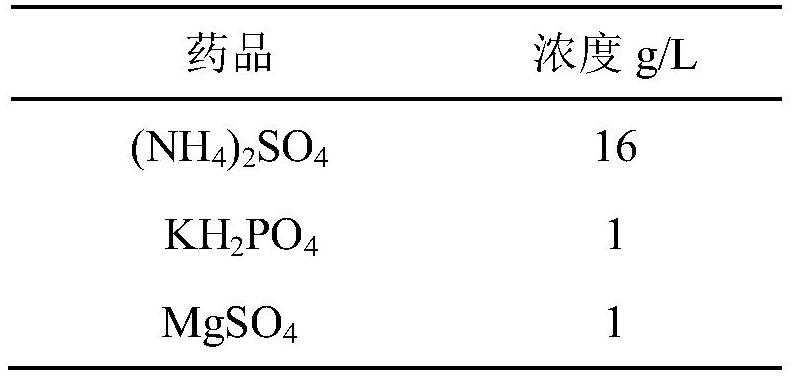
[0028] The strains constructed above were cultured in shake flasks, and the composition of the culture medium was as follows:
[0029] Table 2: Recipe Components of the Medium
[0030]
[0031]
[0032] The broth was neutralized with CaCO by adding 0.5M HCl 3 After that, the OD600 value was measured. Add 50-100 μL ammonia water every 3 hours to adjust the pH ≥ 7. Shake culture at 37°C and 200 rpm until OD600 is about 0.8, add inducer IPTG to a final concentration of 1 mmol L -1 , continue to cultivate for 28 hours, and collect the bacteria by centrifugation.
[0033] 2. Fermentation result:
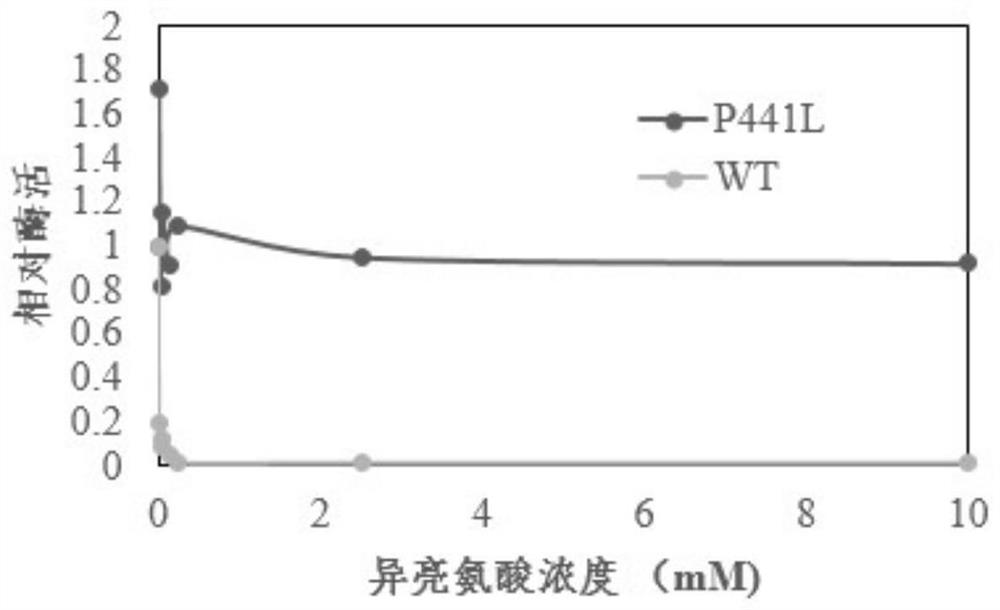
[0034] Under the same fermentation conditions, the growth and sugar consumption of the three strains overexpressing the TD gene are basically the same, but when the residual sugar and threonine are exhausted at 28 hours, the overexpressing mutant TD can produce 3.81g / L isoleucine, while the overe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com