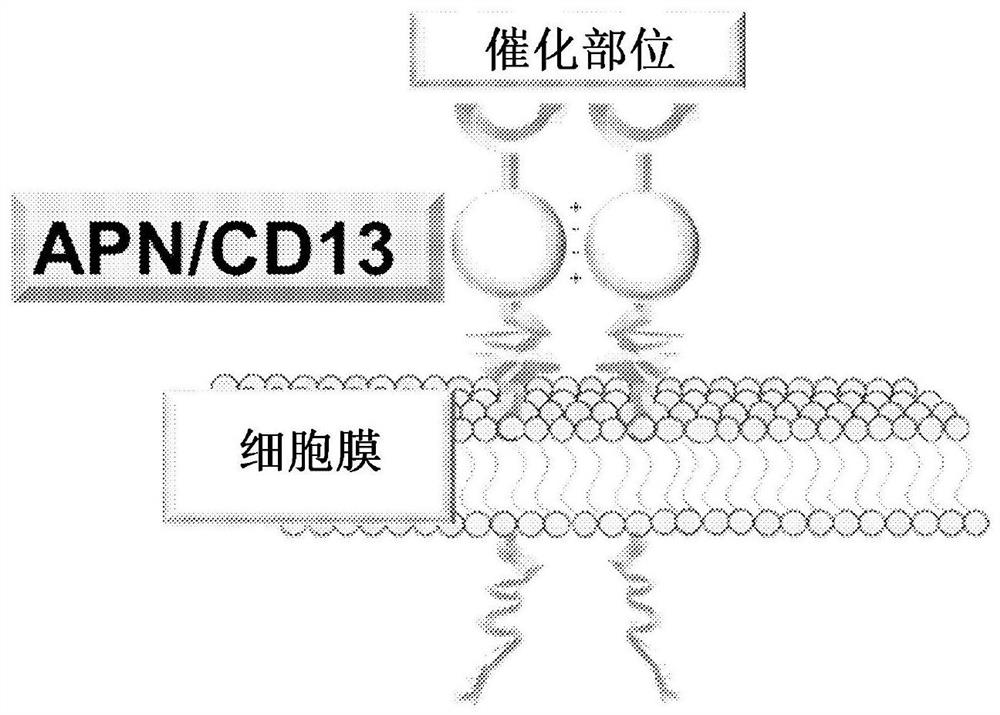
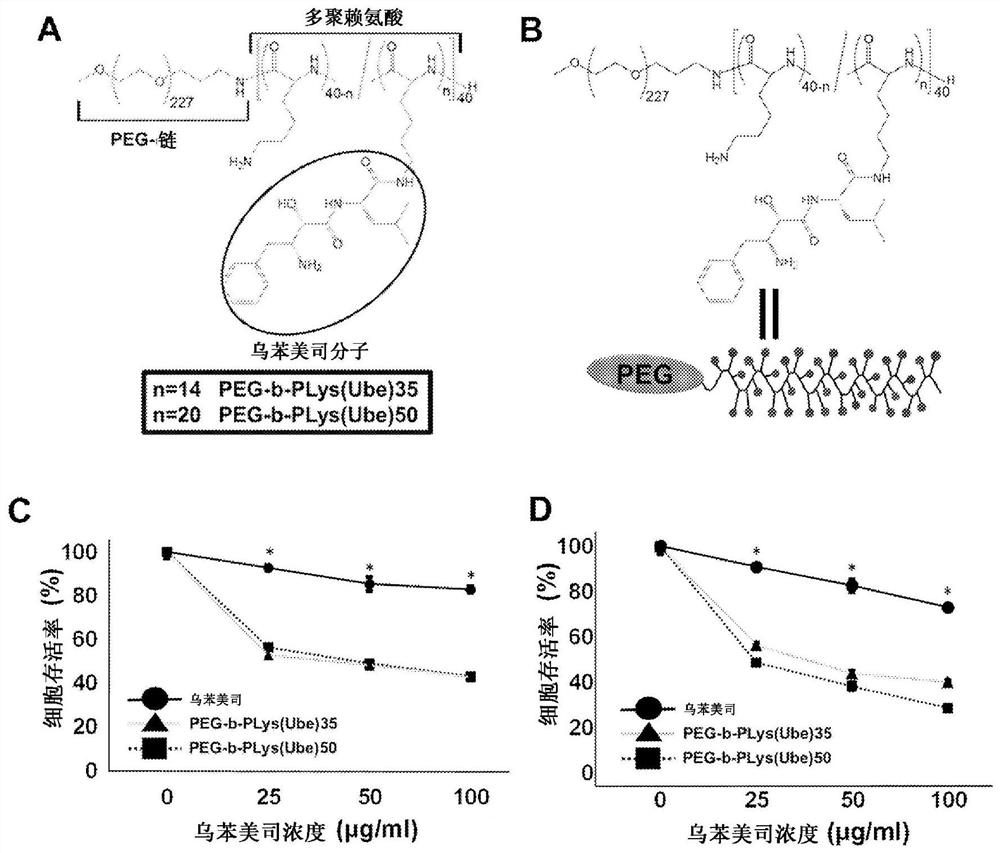
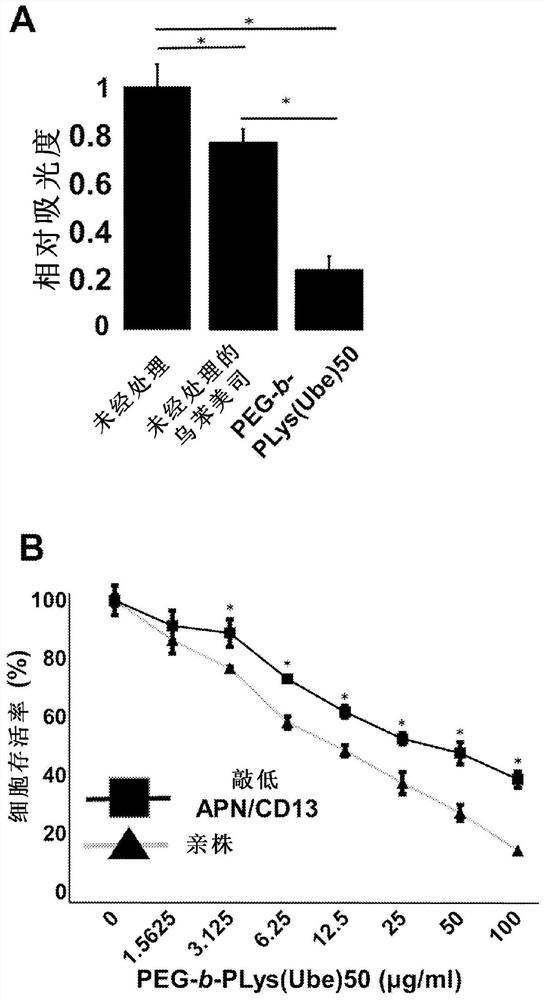
Anticancer agent
A technology of anticancer agents and compounds, which can be used in antitumor drugs, peptide/protein components, drug combinations, etc., and can solve problems such as inability to exert anticancer effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0109] Hereinafter, although this invention is demonstrated in detail based on an Example, this invention is not limited to these Examples.
Synthetic example 1
[0110] Synthesis Example 1. Synthesis of PEG-b-Plys(Ube)20
[0111] A compound comprising a chain structure was synthesized in the following manner by making 20% of lysine residues of a block copolymer of polyethylene glycol and polylysine (PEG-b-Plys) The amino group on the side chain is connected with the carboxyl group of Ubenimex through an amide bond. Specifically, the synthetic figure 2 A and figure 2 A compound of n=8 in the structural formula shown in B.
Synthetic example 1-1
[0112] Synthesis Example 1-1. Synthesis of PEG-b-Plys
[0113] 1.1 g of polyethylene glycol (PEG) having a methoxy group at one end and an amino group at the other end (average molecular weight: 10000) was taken and dissolved in 10 mL of dimethyl sulfoxide. Dissolve 1.3 g of N-(4-(2,5-dioxo-4-oxazolidinyl)butyl)-2,2,2-trifluoroacetamide (Lys(TFA)-NCA) in 10 mL of dimethyl sulfoxide. The resulting two solutions were combined under an atmosphere of argon and stirred overnight at room temperature. Next, the reaction solution was poured into excess diethyl ether so that the product was precipitated again and recovered, and dried under reduced pressure. The obtained white powder was dissolved in 100 mL of a mixed solution of methanol / 1M aqueous NaOH (9 / 1 [v / v]), and stirred overnight at 35°C. The pH of the reaction solution was neutralized to 1-2 with hydrochloric acid. Dialysis was further performed, and a white powder (1.1 g, yield 61%) was obtained by freeze-drying.
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com