Metformin hydrochloride controlled release tablets and preparation method thereof
A technology of metformin hydrochloride and controlled-release tablets, which is used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. The total weight is large and other problems, so as to ensure the clinical effectiveness and reduce the adverse reaction rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
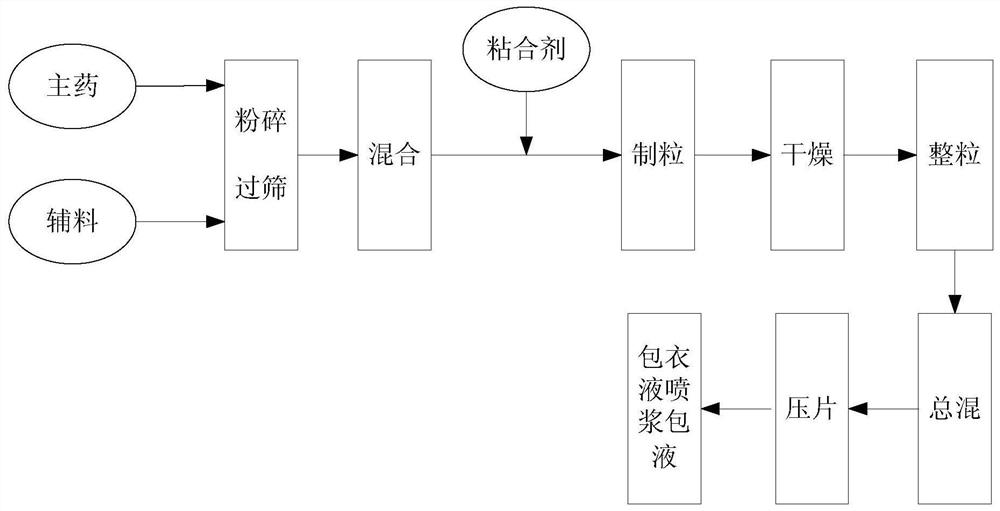
[0042] A kind of preparation method of metformin hydrochloride controlled-release tablet, such as figure 1 shown, including steps:
[0043] (1) The main drug metformin hydrochloride, the auxiliary material filler, the auxiliary material slow-release material are pulverized and sieved, and a binder is added to make a soft material;
[0044] (2) Soft materials are granulated, dried, granulated, and tableted to make tablet cores;
[0045] (3) The tablet core coating solution is sprayed with slurry to form a slow-release coating film outside the tablet core;
[0046]In step (1), the sustained-release material is a mixture of stearyl alcohol and polyethylene oxide, and its proportioning is in parts by weight: stearyl alcohol: polyethylene oxide=3:1, and the molecular weight of polyethylene oxide is 400,000 to 900,000; the ratio of the main drug metformin hydrochloride to the auxiliary material sustained-release material is in parts by weight: metformin hydrochloride: sustained-re...
example 1
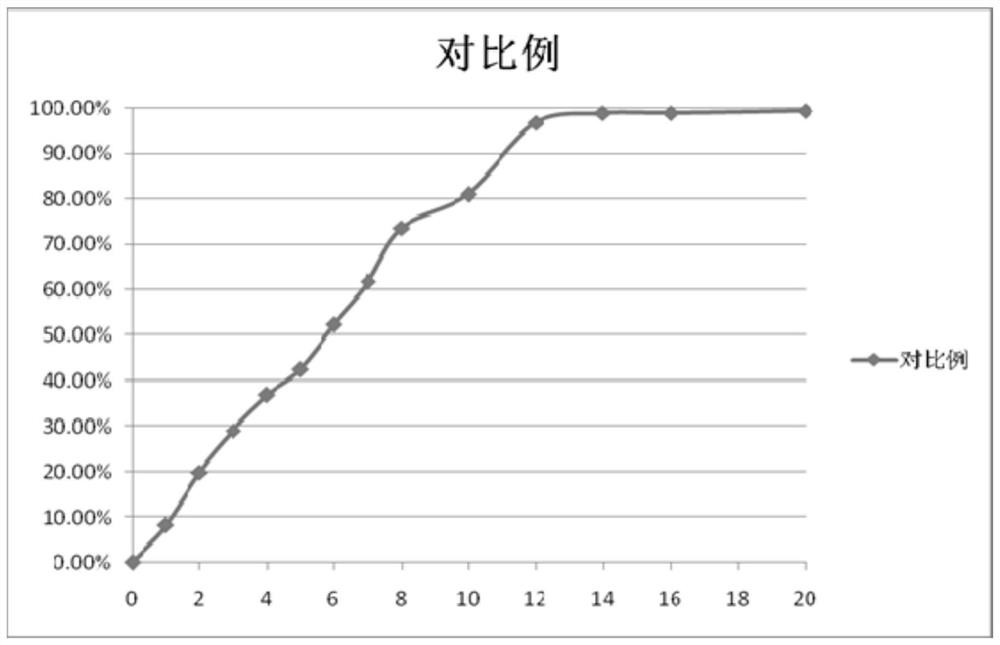
[0053] Metformin hydrochloride controlled-release tablets were prepared according to the weight ratio of each component in Table 1.
[0054] Table 1
[0055]
[0056] The preparation method of the metformin hydrochloride controlled-release tablet of this example comprises steps:
[0057] (1) The main drug metformin hydrochloride, the auxiliary material filler and the auxiliary material slow-release material are pulverized and sieved, and a binder is added to make a soft material.
[0058] In this example, lactose is used as the filler for the auxiliary material; the slow-release material for the auxiliary material is a mixture of stearyl alcohol and polyethylene oxide, and the proportioning is in parts by weight: stearyl alcohol:polyethylene oxide=3:1 , the polyethylene oxide molecular weight is 900,000; the adhesive uses povidone K30, and the povidone K30 is configured as a 5% aqueous solution. Take out metformin hydrochloride, lactose, stearyl alcohol, and polyethylene ...
example 2
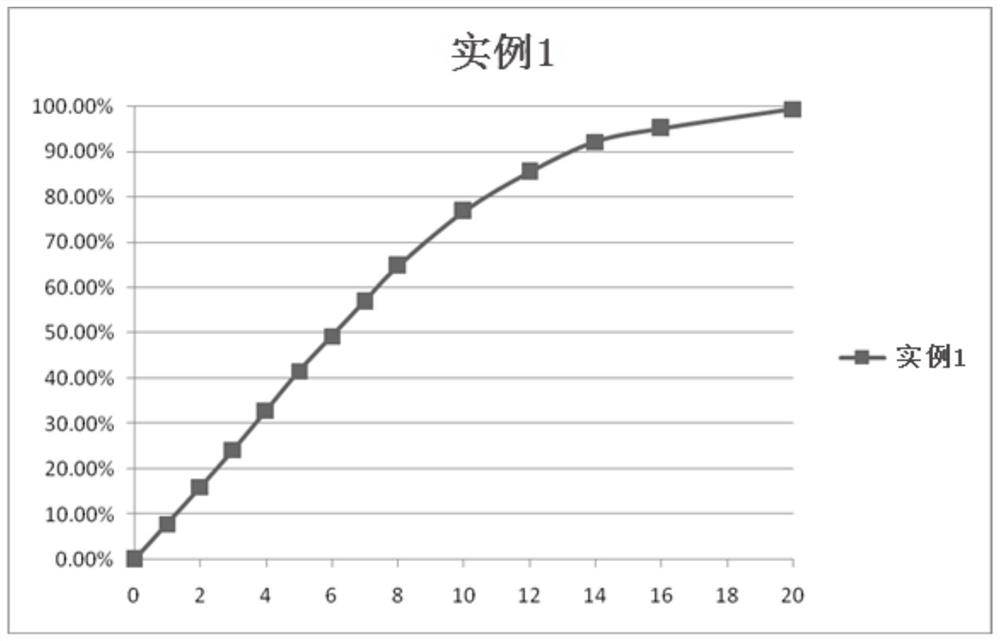
[0065] Metformin hydrochloride controlled-release tablets were prepared according to the weight ratio of each component in Table 2.
[0066] Table 2
[0067]
[0068]
[0069] The preparation method of the metformin hydrochloride controlled-release tablet of this example comprises steps:
[0070] (1) The main drug metformin hydrochloride, the auxiliary material filler and the auxiliary material slow-release material are pulverized and sieved, and a binder is added to make a soft material.
[0071] In this example, the filler of the auxiliary material adopts pregelatinized starch; the slow-release material of the auxiliary material adopts a mixture of stearyl alcohol and polyethylene oxide, and its proportioning is in parts by weight: stearyl alcohol: polyethylene oxide = 3:1, the polyethylene oxide molecular weight is 900,000; the binder uses hypromellose (SH-E5), and the hypromellose (SH-E5) is configured as a 3% aqueous solution . Take out metformin hydrochloride, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com