Method for promoting arginine to quickly release NO by black phosphorus
A technology of arginine and black phosphorus, which is applied in the directions of biochemical equipment and methods, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve the problems of slow release and low release amount, etc. Good stability and good photothermal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis and Characterization of BP-Arg-GOx
[0032] (1) Synthesis of BP-Arg: Measure 2 mL of 1 mg / mL black phosphorus in DMF (or dimethyl sulfoxide) solution, add 6 mg of arginine (you can also use canavanic acid and other guanidine groups that can be oxidized to produce NO) compound) and 3 mg DMAP, and then 200 μL of deionized water was added, and the mixture was reacted overnight for 8 h. After the reaction was completed, the mixed solution was dialyzed for 6 hours to remove unreacted arginine. After the dialysis, the obtained product was centrifuged at 17,000 g for 4 minutes, washed three times with deionized water, and finally the obtained product was resuspended in deionized water for storage.
[0033] (2) Synthesis of BP-Arg-GOx: Weigh 2mg GOx, add 5mg EDC and 10mg NHS, activate the carboxyl group on GOx in PB, add 5mg BP-Arg to the activated GOx after 30min, react overnight, centrifuge Take the pellet and resuspend it in PB for subsequent use.
[0034] BP-Arg...
Embodiment 2
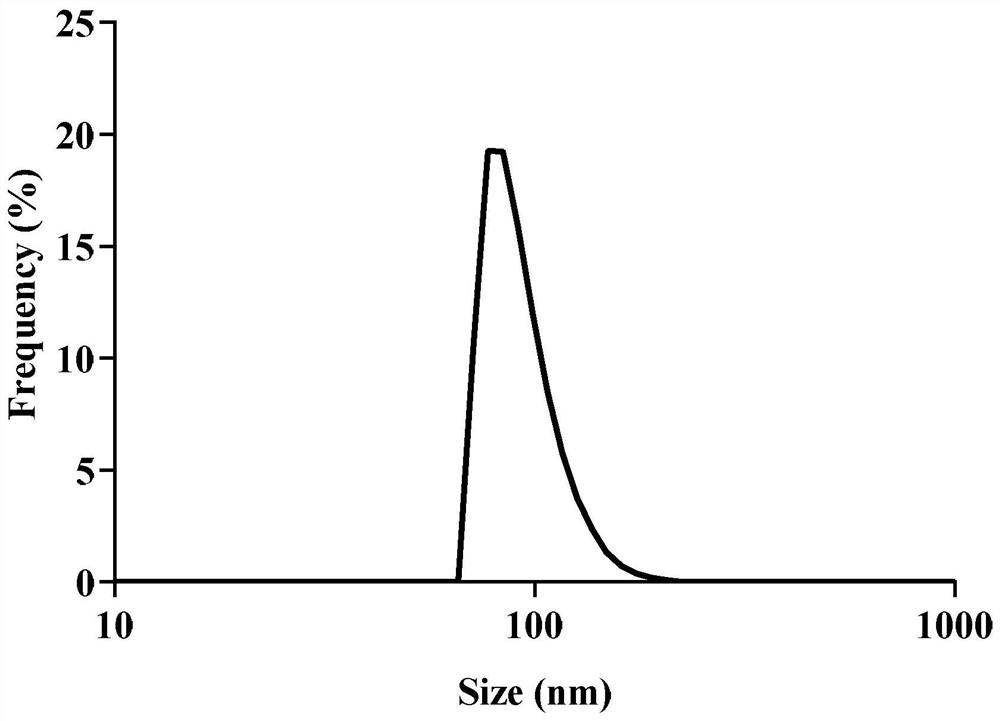
[0036] Determination of particle size spectrum: Take appropriate amount of black phosphorus and BP-Arg-GOx respectively, and measure their hydrodynamic diameters in PB 7.4 respectively. In addition, the hydrodynamic diameter of BP-Arg was measured on the 1st, 5th, and 10th day to monitor its stability.
Embodiment 3
[0038] Determination of NO release profile: take a certain amount of BP-Arg-GOx, and measure its NO release.
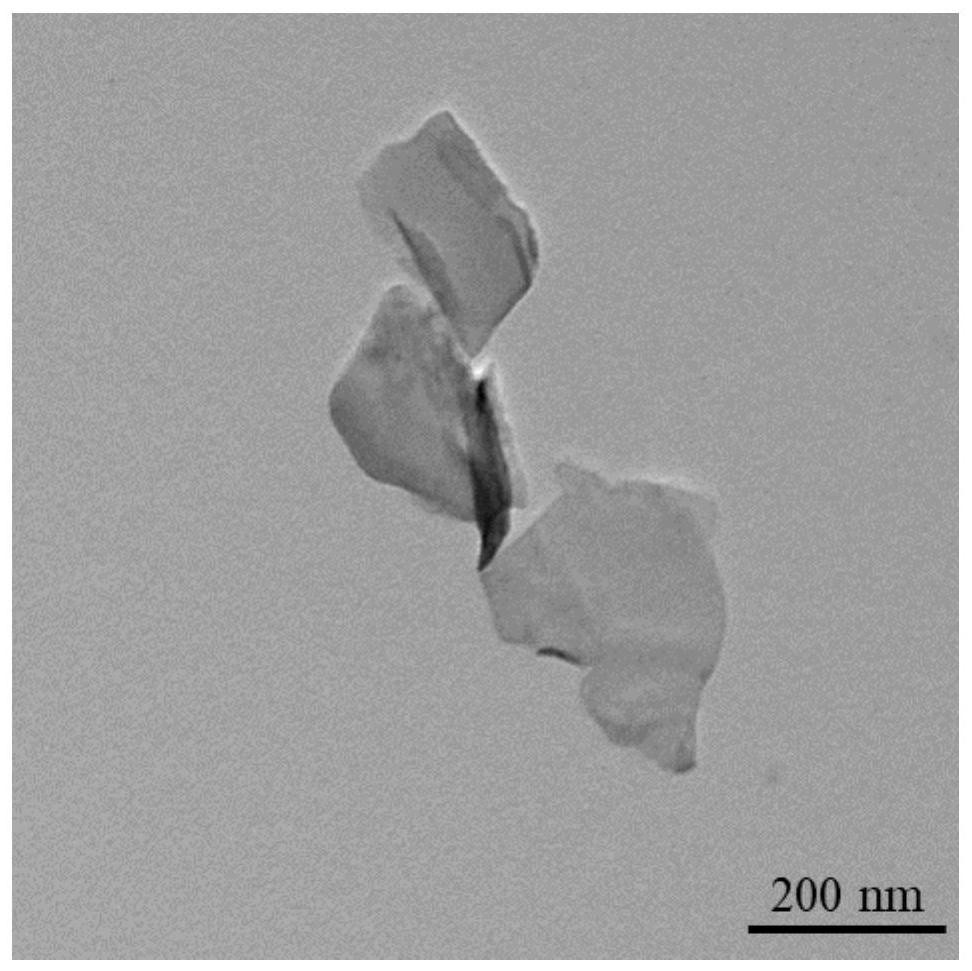
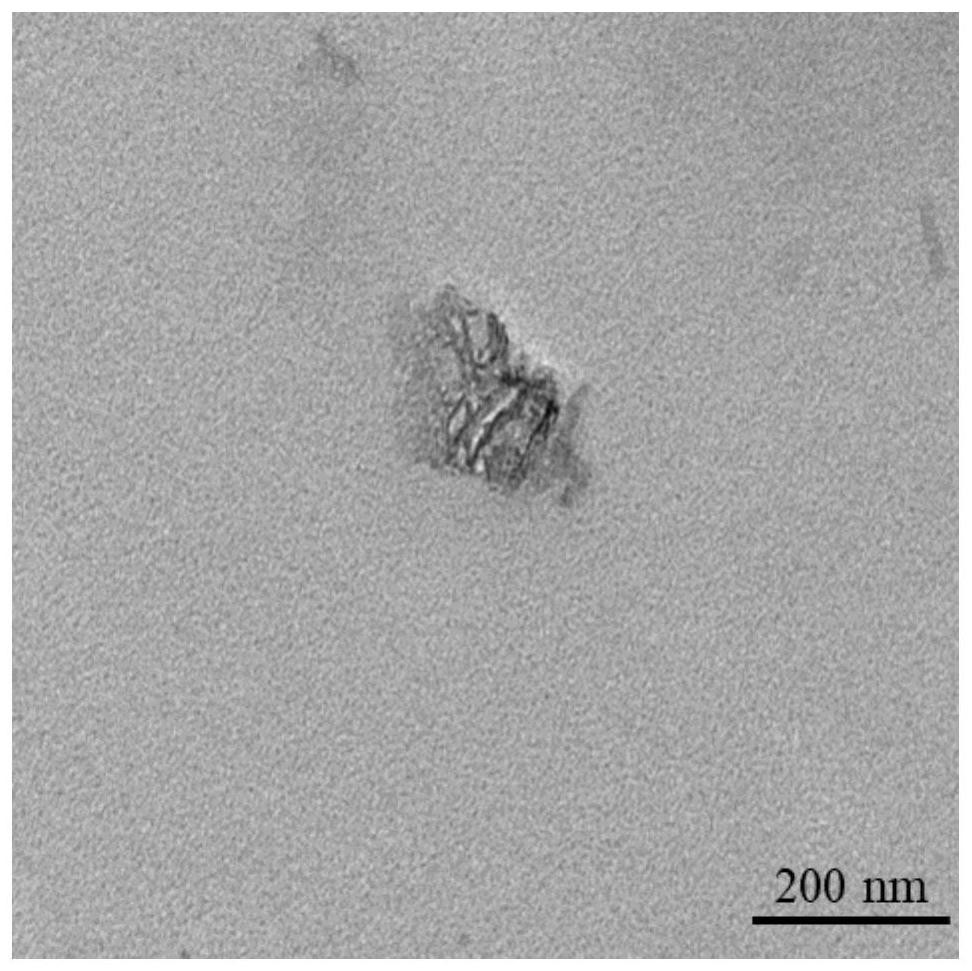
[0039] as attached Figure 1-4 As shown, the modified product BP-Arg-GOx and the raw material BP were analyzed by TEM and DLS. The surface of the modified BP showed a significant difference from the raw material BP. The DLS results also proved that BP-Arg-GOx compared with BP , the particle size increases to a certain extent.
[0040] as attached Figure 5 As shown, the DLS analysis of the modified product BP-Arg-GOx shows that BP-Arg-GOx still maintains good stability after 5 days in PBS solution.
[0041] as attached Figure 6 As shown, the modified product BP-Arg-GOx showed similar enzyme activity to free GOx after NIR irradiation, indicating the success of BP-Arg-GOx modification and the maximum retention of enzyme activity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com