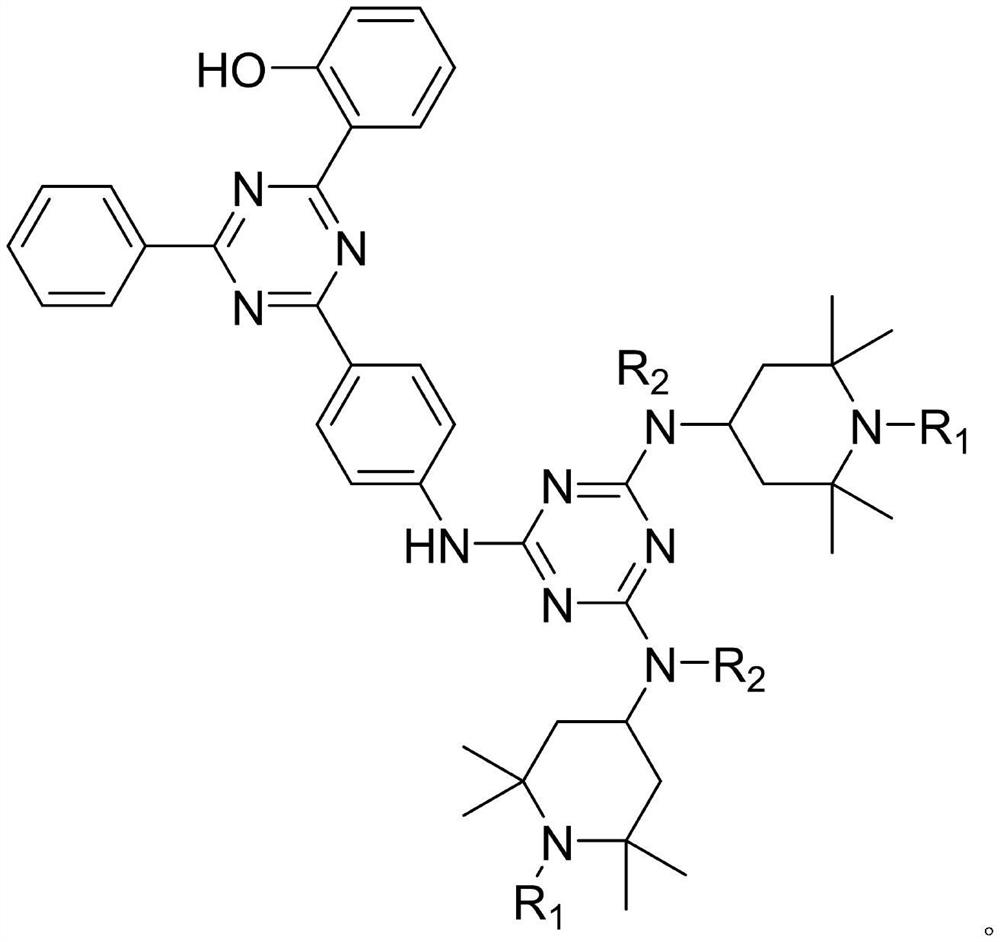
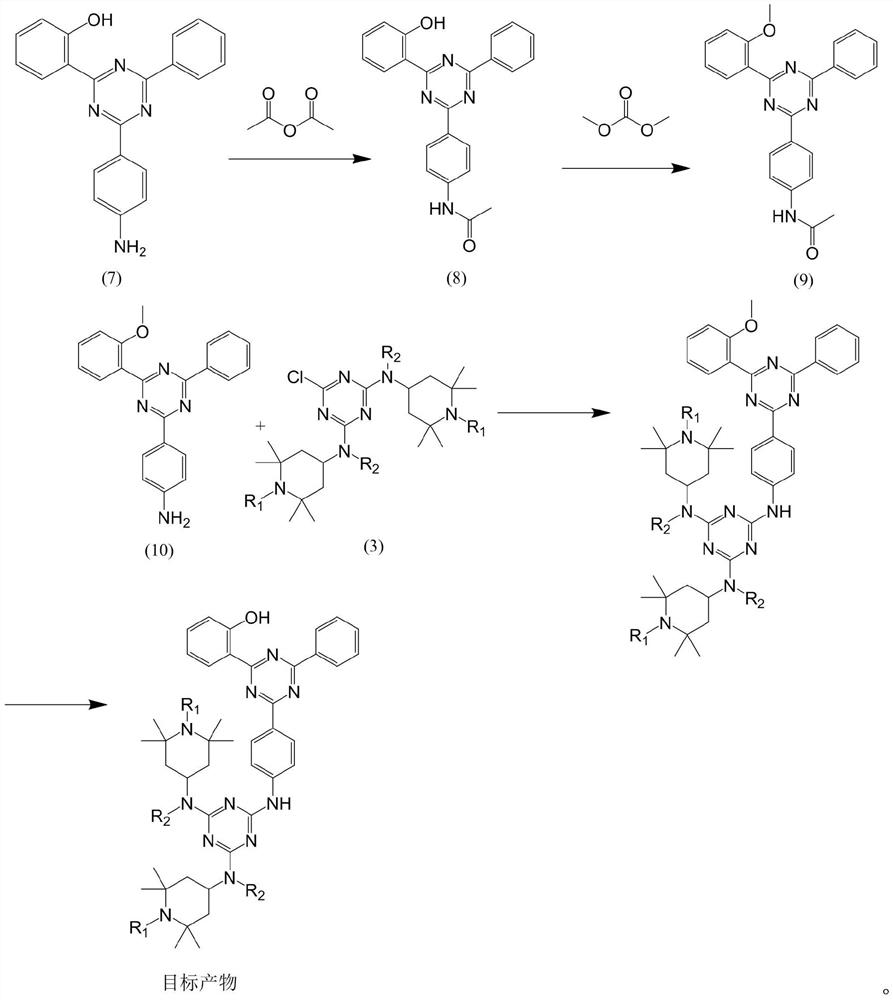
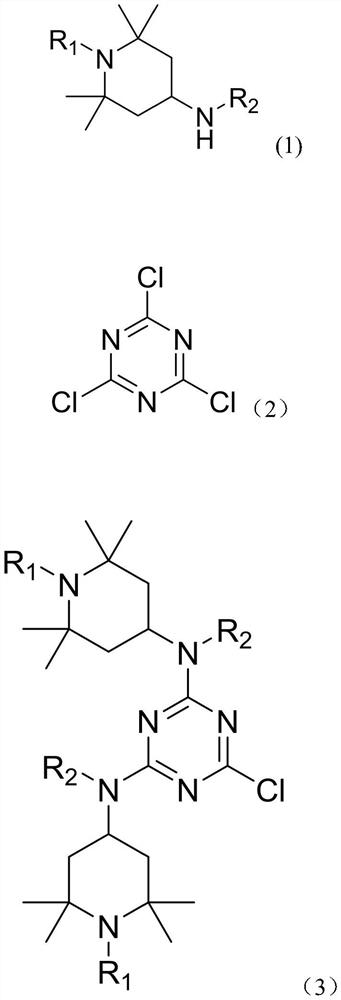
Preparation method of multifunctional light stabilizer
A light stabilizer and multifunctional technology, which is applied in the field of multifunctional light stabilizers and preparations, can solve the problems of triazine compounds such as heavy color, yellowing of materials, and limitations, and achieve the effect of excellent ultraviolet absorption spectral characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] a) Take a dry 500ml three-necked round-bottom flask, install a thermometer on the left side, install a condensing reflux device in the middle, and install a constant pressure dropping funnel on the right side, add ZJ-702 (10.00g), cyclohexyl formaldehyde (6.58g), ethanol 20ml, stir and dissolve at room temperature, add o-phenanthroline copper complex (5mol%), slowly drop 10ml 30% hydrogen peroxide aqueous solution, after the dropwise addition, heat the oil bath to 35-45°C, and react for 8h. The solvent was distilled off under reduced pressure, and the pure compound N-cyclohexyloxytetramethylpiperidone was obtained as a white foamy solid through column chromatography.
[0038] b) Put the product obtained in step a into a 500ml single-necked round bottom flask, add 50ml of absolute ethanol as a solvent, add 5g of activated 4A-molecular sieve, stir, slowly add an equimolar amount of n-butylamine dropwise and assemble a condenser , heated in an oil bath, refluxed, and TLC d...
Embodiment 2
[0046] a) Take a dry 500ml three-necked round-bottom flask, install a thermometer on the left, install a condensing reflux device in the middle, and install a constant pressure dropping funnel on the right, add ZJ-702 (10.00g), cyclohexene (4.92g), ethanol 20ml, stir and dissolve at room temperature, add 2,2'-bipyridine copper complex (5mol%), slowly drop 10ml 30% hydrogen peroxide aqueous solution, after the dropwise addition, heat the oil bath to 35-45°C, and react for 8h . The solvent was distilled off under reduced pressure, and the pure compound N-cyclohexyloxytetramethylpiperidone was obtained as a white foamy solid through column chromatography.
[0047] b) the product obtained in step a is dropped into a 500ml single-necked round-bottomed flask, add 50ml of absolute ethanol as solvent, add 4g of anhydrous sodium sulfate, stir, and slowly add dropwise an equimolar amount of n-butylamine to assemble a condenser. Oil bath heating, reflux, TLC detects that the reaction pr...
Embodiment 3
[0052] a) Take a dry 200ml single-necked round bottom flask and add ZJ-702 (10.00g), sodium ascorbate (11.65g) and 50ml of water. After stirring at room temperature, a suspension appeared, which was then extracted with dichloromethane. Dry and remove solvent. 1-Hydroxy-2,2,6,6,-tetramethylpiperidin-4-one was obtained as a white solid.
[0053] b) The product obtained in step a is put into a 500ml single-necked round-bottomed flask, and 50ml of absolute ethanol is added as a solvent, stirred, slowly added dropwise in an equimolar amount of n-butylamine and assembled with a Dean-Stark device. Oil bath heating, reflux, TLC detects that the reaction proceeds, and after the reaction finishes, the solvent is distilled off under reduced pressure to obtain an intermediate product, an oil.
[0054] c) The product obtained in the step b is added to a 200ml autoclave, 50ml hexanaphthene is added, and 5mol% palladium carbon catalyst is added. Filled with 1.5MPa H 2 , reacted at 60-120...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com