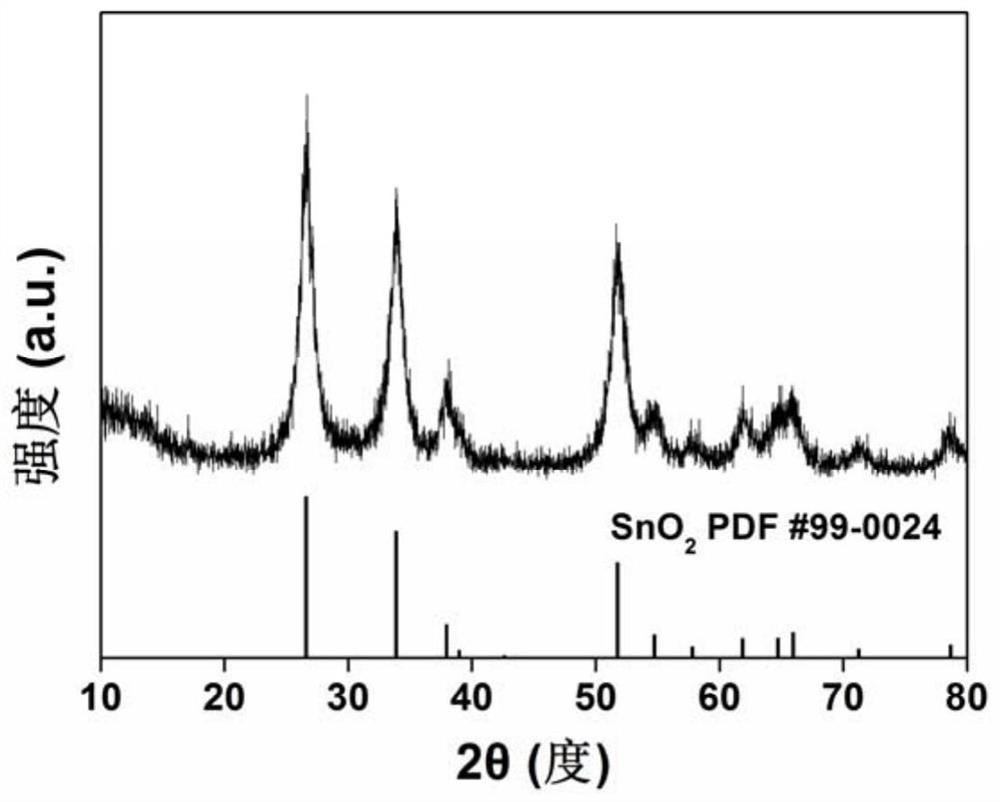
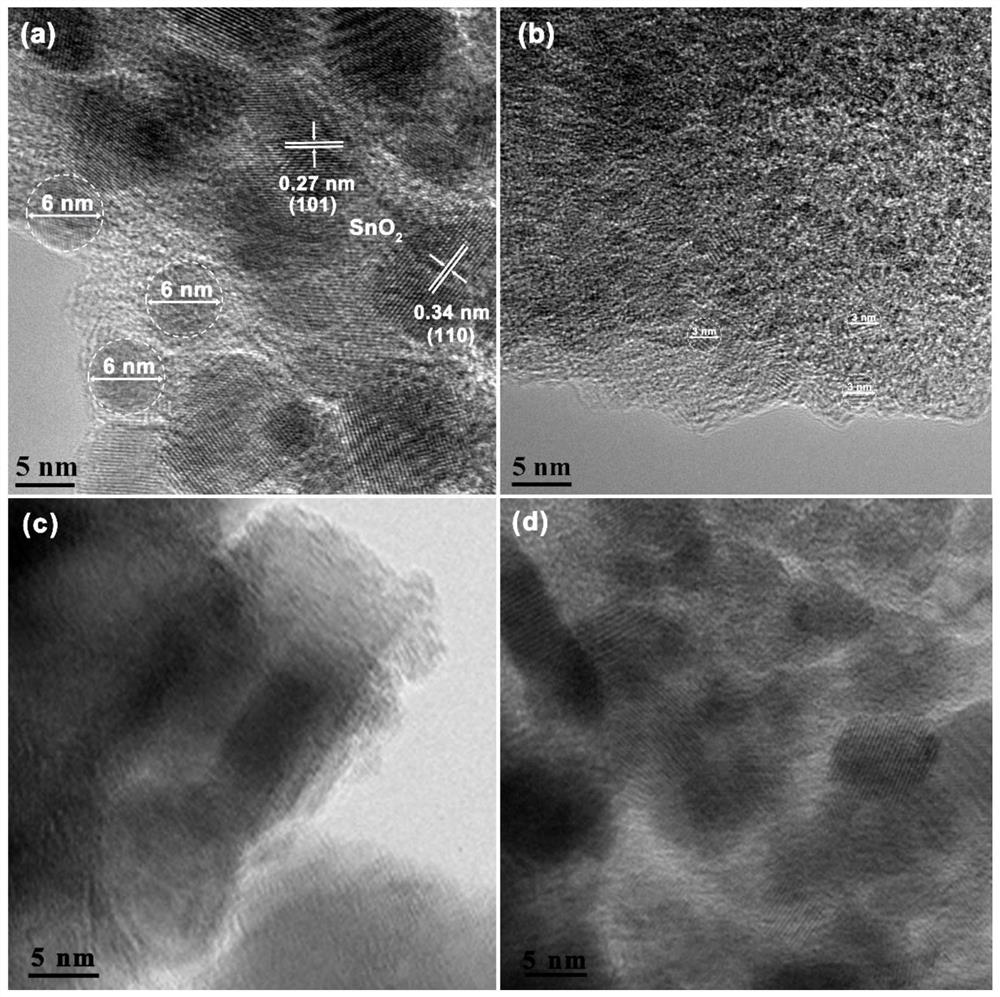
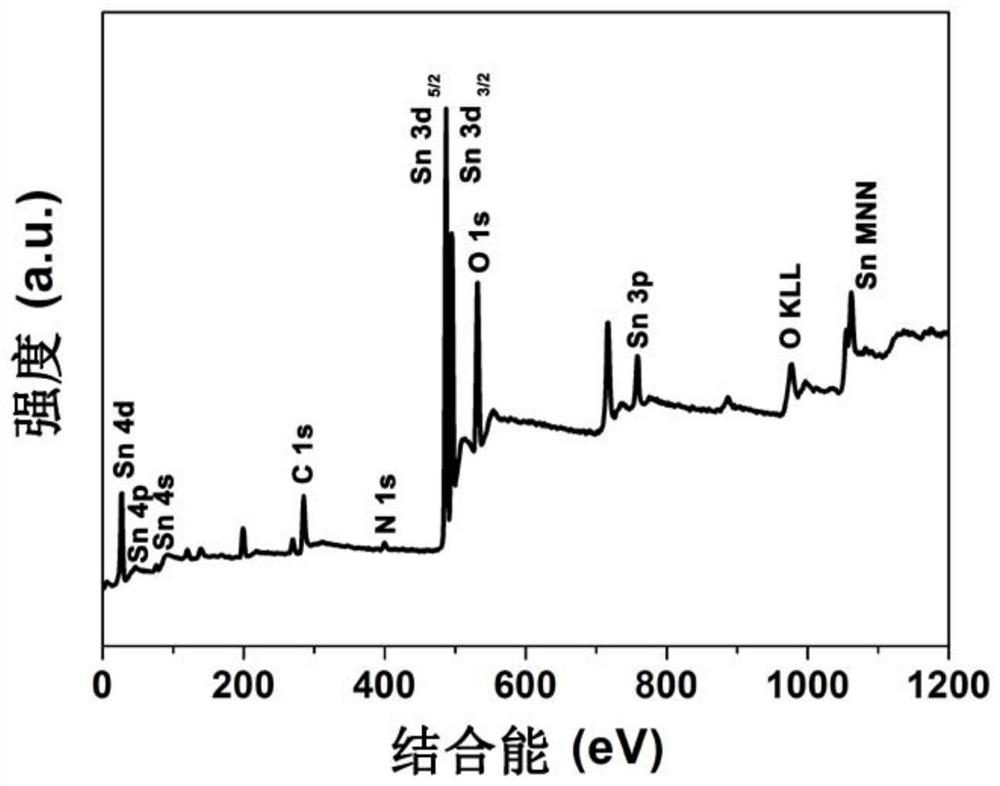
Nitrogen-doped carbon nano SnO2 composite material and preparation method and application thereof
A composite material, nitrogen-doped carbon technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve problems such as complex preparation methods, achieve simple preparation methods, wide application prospects, repeatable good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1) Preparation of poly-4-vinylpyridine / absolute ethanol solution: at room temperature, get 5.25g poly-4-vinylpyridine (weight average molecular weight 60000) and join in 50mL of absolute ethanol, and magnetic stirring makes it dissolve, obtains 4 -Poly(4-vinylpyridine) / absolute ethanol solution with a vinylpyridine monomer concentration of 1 mol / L.
[0054] (2) Preparation of SnCl 2 2H 2 O / absolute ethanol solution: take 11.28g SnCl 2 2H 2 O was added to 50 mL of absolute ethanol, and magnetically stirred to dissolve it to obtain SnCl with a concentration of 1 mol / L 2 2H 2 O / absolute ethanol solution.
[0055] (3) Preparation of carbonized precursor: under the condition of magnetic stirring at room temperature, the SnCl 2 2H 2 O / dehydrated ethanol solution is added in poly-4-vinylpyridine / dehydrated ethanol solution, molar ratio (Sn 2+ : 4-vinylpyridine monomer = 1:1); continue to stir for 12 hours to form a stable complex precipitate, and then evaporate and ...
Embodiment 2
[0058] (1) Preparation of poly-4-vinylpyridine / absolute ethanol solution: at room temperature, get 5.25g poly-4-vinylpyridine (weight average molecular weight 60000) and join in 50mL of absolute ethanol, and magnetic stirring makes it dissolve, obtains 4 -Poly(4-vinylpyridine) / absolute ethanol solution with a vinylpyridine monomer concentration of 1 mol / L.
[0059] (2) Preparation of SnCl 2 2H 2 O / absolute ethanol solution: take 2.82g SnCl 2 2H 2 O was added to 50 mL of absolute ethanol, and magnetically stirred to dissolve it to obtain SnCl with a concentration of 0.25 mol / L 2 2H 2 O / absolute ethanol solution.
[0060] (3) Preparation of carbonized precursor: under the condition of magnetic stirring at room temperature, the SnCl 2 2H 2 O / dehydrated ethanol solution is added in poly-4-vinylpyridine / dehydrated ethanol solution, molar ratio (Sn 2+ : 4-vinylpyridine monomer = 1:4); continue to stir for 12 hours to form a stable complex precipitate, and then evaporate an...
Embodiment 3
[0063] (1) Preparation of 4-vinylpyridine monomer / absolute ethanol solution: at room temperature, take 5.25g of 4-vinylpyridine monomer and add it to 50mL of absolute ethanol, and stir it magnetically to dissolve it to obtain 4-vinylpyridine monomer 4-vinylpyridine monomer / absolute ethanol solution with a concentration of 1mol / L.
[0064] (2) Preparation of SnCl 2 2H 2 O / absolute ethanol solution: take 11.28g SnCl 2 2H 2 O was added to 50 mL of absolute ethanol, and magnetically stirred to dissolve it to obtain SnCl with a concentration of 1 mol / L 2 2H 2 O / absolute ethanol solution.
[0065] (3) Preparation of carbonized precursor: under the condition of magnetic stirring at room temperature, the SnCl 2 2H 2 O / dehydrated ethanol solution is added in 4-vinylpyridine monomer / dehydrated ethanol solution, molar ratio (Sn 2+ : 4-vinylpyridine monomer = 1:1); continue to stir for 12 hours to form a stable complex precipitate, and then evaporate and dry to obtain the carbon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Reversible capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com