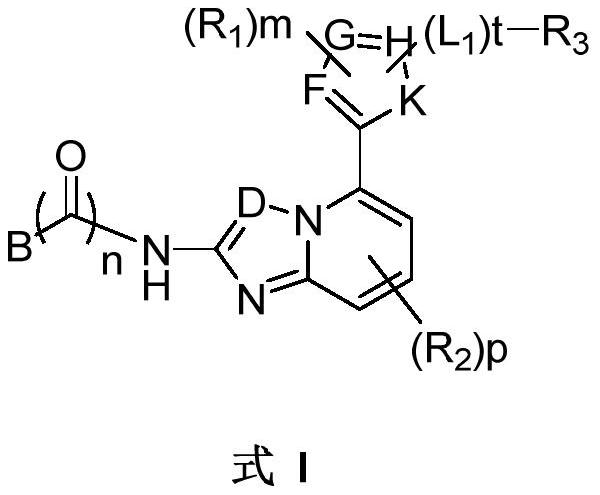
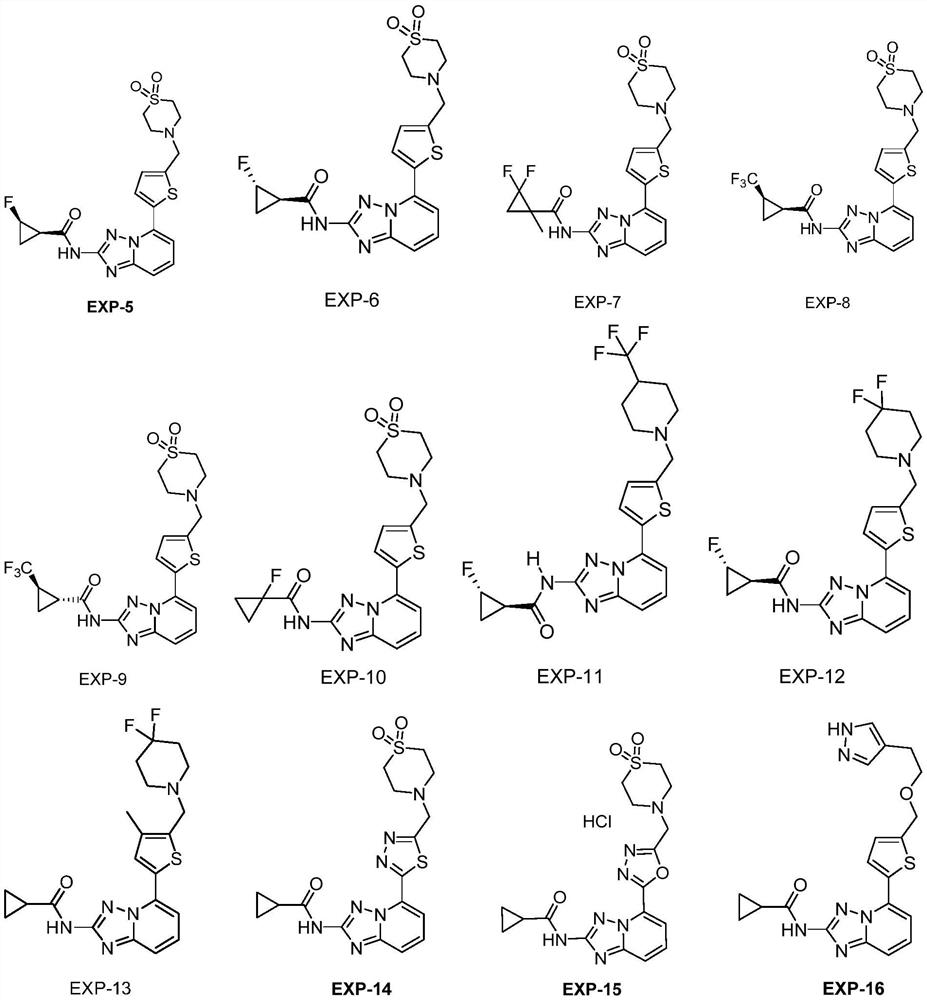
JAK kinase inhibitor and application thereof
A solvate, selected technology, applied in the field of medicine, can solve the problem of loss, painful movement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
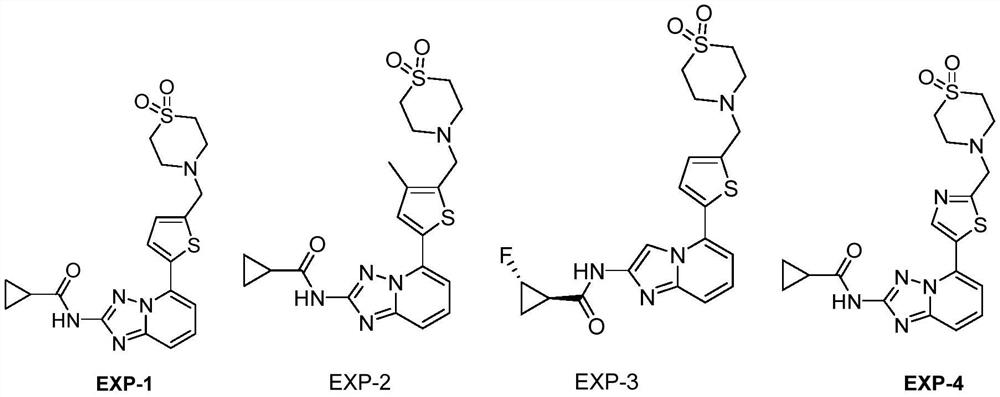
[0076] Embodiment 1: the preparation of compound EXP-1
[0077] The synthetic route is as follows:
[0078]
[0079] Step 1.1 Preparation of compound 1
[0080]
[0081] 5-Bromo-[1,2,4]triazolo[1,5-a]pyridin-2-amine (SM1) (2 g, 9.4 mmol) and triethylamine (2.4 g, 23.5 mmol) were dissolved in acetonitrile ( 10 mL), cyclopropylformyl chloride (SM2) (2.45 g, 23.5 mmol) was added dropwise at 0° C., and the mixture was naturally raised to room temperature and stirred for 16 hours. The reaction solution was concentrated in vacuo to obtain the bisacylated intermediate N-(5-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-yl)-N-(cyclopropanecarbonyl)cyclopropane Formamide (1) (3.3 g crude, yellow solid) was used in the next step without further purification.
[0082] LCMS:t R =0.785min in 5-95AB_1.5min_220&254_Shimadzu.lcmchromatography (Agilent Pursult 5C18 20*2.0mm), MS (ESI) m / z=350.9[M+2+H] + .
[0083] Step 1.2 Preparation of Compound Int-1
[0084]
[0085] N-(5-Bromo-[1,2,...
Embodiment 2
[0101] Embodiment 2: the preparation of compound EXP-3
[0102] The synthetic route is as follows:
[0103]
[0104] Step 2.1 Preparation of compound 22
[0105]
[0106] 6-Bromopyridin-2-amine (SM16) (5 g, 28.90 mmol) and ethyl bromopyruvate (SM17) (6.20 g, 31.79 mmol) were dissolved in ethanol (30 mL), and stirred at 85 degrees for 16 hours. The reaction solution was filtered, and the filter cake was collected, slurried with petroleum ether / ethyl acetate (5 / 1, v / v, 50mL), solid filtered, and dried to obtain 5-bromoimidazole[1,2-a]pyridine-2-carboxylic acid Ethyl ester (22) (8.41 g, 83% yield, hydrogen bromide salt, yellow solid).
[0107] 1 H NMR (400MHz, DMSO-d 6 ):δ=8.56(s,1H),7.75(d,J=8.8Hz,1H),7.52-7.44(m,2H),4.36(q,J=7.2Hz,2H),1.34(t,J= 7.2Hz, 3H).
[0108] Step 2.2 Preparation of compound 23
[0109]
[0110] Dissolve ethyl 5-bromoimidazol[1,2-a]pyridine-2-carboxylate (22) (2 g, 5.71 mmol, hydrogen bromide salt) in water, 5 ml each of tetrahydrofuran and ...
Embodiment 3
[0135] Embodiment 3: the preparation of compound EXP-4
[0136] The synthetic route is as follows:
[0137]
[0138] Step 3.1 Preparation of compound 3
[0139]
[0140] Dissolve 2-methylthiazol-5-boronate (SM5) (100mg, 0.444mmol) in carbon tetrachloride (2mL), add N-bromosuccinimide (103mg, 0.577mmol) and azobisisobutyronitrile (7.3 mg, 0.0444 mmol). The reaction solution was stirred at 80° C. for 0.5 hour under the protection of nitrogen. The reaction solution was filtered, and the filtrate was concentrated in vacuo to obtain a crude product, which was then dissolved in tetrahydrofuran (2mL), and diisopropylethylamine (0.1mL) and diethyl phosphite (74uL) were added successively, and stirred at 10°C for 25 minutes to obtain 2- Bromomethylthiazole-5-boronate (3) (135 mg, crude, brown solution in 2 mL THF) was used directly in the next step.
[0141] LCMS:t R =0.590min in 5-95AB_1.5min_220&254_Shimadzu.lcmchromatography(Merk RP18e 25-3mm), MS(ESI)m / z=221.7[M-81] + ....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com