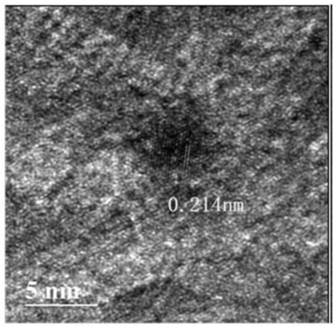
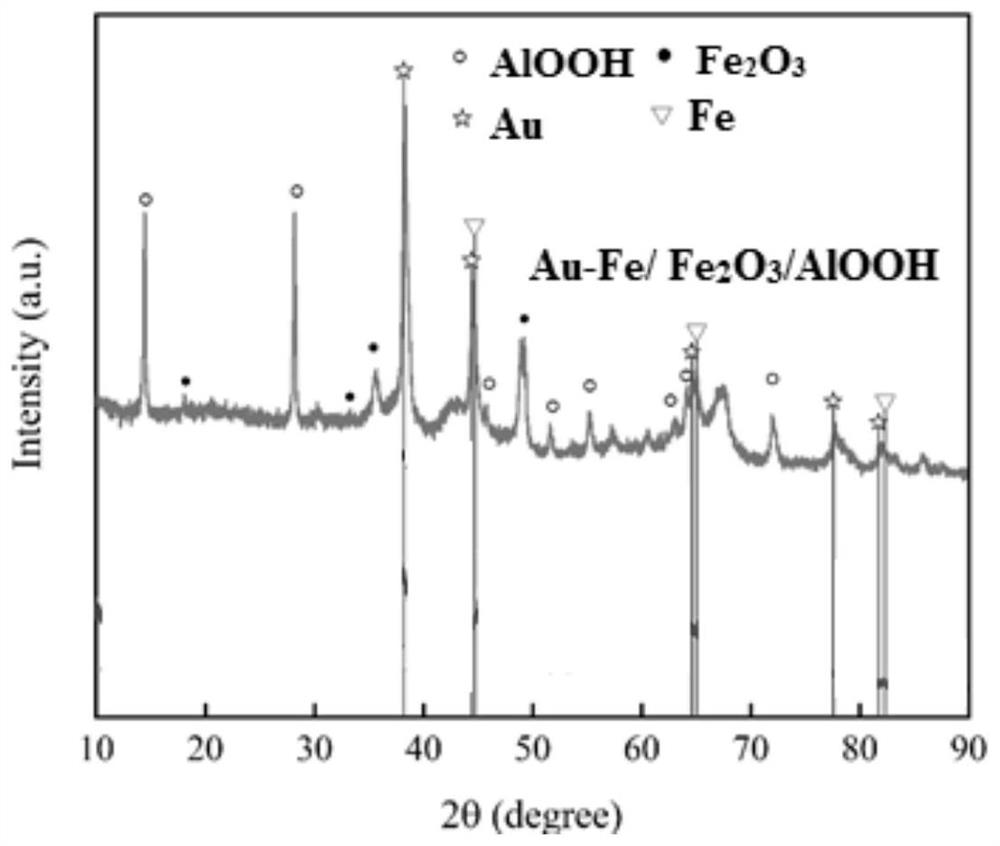
Gold-based catalyst, and preparation method and application thereof
A catalyst and gold-based technology, which is applied to the gold-based catalyst for preparing methacrolein dimethyl acetal and the field of preparation thereof, can solve the problem of low selectivity of MDA, low conversion rate of methacrolein, and inability to realize methyl acrolein at the same time. Problems such as high conversion of acrolein and high selectivity of MDA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The invention provides a kind of preparation method of gold-based catalyst, comprises the following steps:
[0034] S1: Dissolve the iron salt and protective agent in the solvent, stir evenly, then add the precipitant and stir evenly to obtain the first suspension. In the present invention, the iron salt is ferric nitrate, ferrous nitrate, ferric sulfate and ferrous sulfate One or more of them, using a precipitant to precipitate the iron ions in the iron salt in the form of hydroxide;
[0035] S2: Add reducing agent solution and carrier to the first suspension, stir evenly and transfer to a reaction kettle for reaction, the reaction temperature is 10-40°C, the reaction time is 8-36h, and the second suspension is obtained, wherein The carrier is aluminum hydroxide; in the presence of a reducing agent, iron ions are reduced to exist as a part of elemental iron;
[0036] S3: washing, filtering, and drying the second suspension to obtain a first solid powder. At this time,...
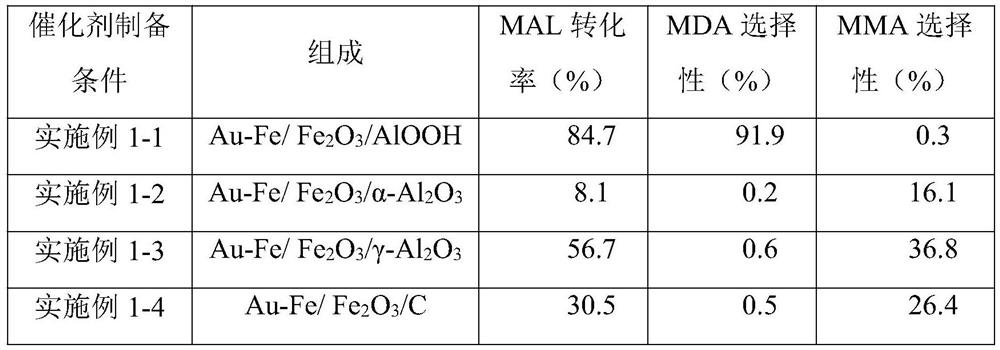
Embodiment 1-1
[0053] Weigh 0.469g Fe(NO 3 ) 3 9H 2 O and 0.50g protective agent were added to a 250mL Erlenmeyer flask, and dissolved in a mixed solvent of 82.5mL deionized water and 12.5mL ethanol, and magnetically stirred for 10min; then 12.5mL of 0.1mol / L NaOH precipitant solution was added to the above Stir in the solution for 10 minutes to obtain the first suspension. In the present invention, the precipitating agent precipitates the iron ions in the iron salt in the form of hydroxide.
[0054] Use a pipette to pipette 25 mL of hydrazine hydrate solution, add it to the above-mentioned first suspension and stir for 10 min; weigh 1 g of carrier AlOOH in a conical flask (the carrier AlOOH contains almost no chlorine in this example), and continue stirring for 10 min. Transfer the solution in the Erlenmeyer flask to the reaction kettle for stirring reaction, the reaction temperature is 25°C, and the reaction time is 18h. After the reaction is completed, the second suspension is obtained,...
Embodiment 1-2
[0060] The difference between this embodiment 1-2 and embodiment 1-1 is that the carrier is α-Al 2 o 3 , other preparation processes are the same as in Example 1-1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com