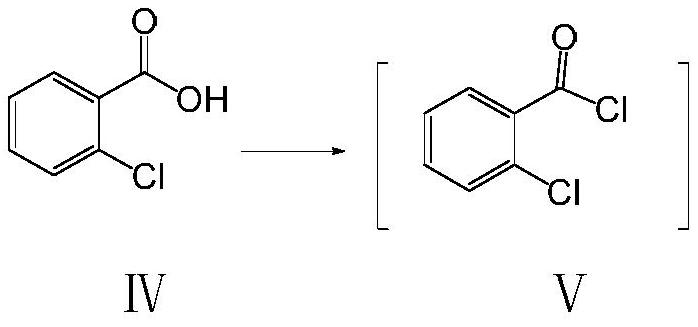
Preparation method of 5-bromo-2-chlorobenzoic acid
A chlorobenzoic acid, chlorination reaction technology, applied in the preparation of carboxylate, the preparation of carboxylic acid amide, the preparation of organic compounds and other directions, can solve the problems of unsuitable industrial production, affecting production and application, increasing production cost, etc., and achieving high industrialization Prospects, improved safety, effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
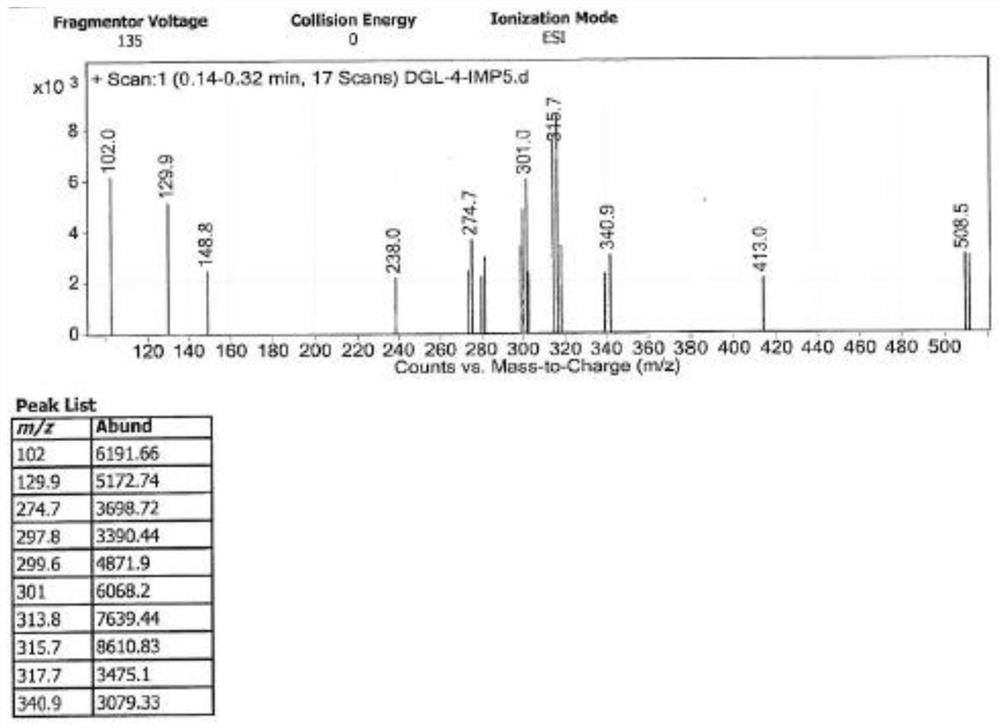
Image
Examples
Embodiment 1
[0033] The preparation method of 5-bromo-2-chlorobenzoic acid in the present embodiment may further comprise the steps:
[0034] (1) Preparation of Compound III
[0035] Add 300mL of toluene, 40.0g of o-chlorobenzoic acid, 22mL of thionyl chloride, and 0.5mL of DMF into a 500mL single-port reaction flask, stir at room temperature, slowly raise the temperature to 70°C, stop heating after four hours of heat preservation, and cool down to room temperature; the reaction system is concentrated To dryness, add 100mL of dichloromethane; take another 500mL four-neck reaction flask, add 25g of 2-amino-2methyl-propanol, 100mL of dichloromethane, 20g of triethylamine, cool to -10°C, add dropwise The solution in the above-mentioned single-necked bottle was kept at a temperature lower than 0°C during the dropwise addition, and reacted at room temperature for 2 hours after the dropwise addition was completed;
[0036] Use 0.01moL of hydrochloric acid to adjust the pH to 5.5~6.5, separate l...
Embodiment 2
[0042] The preparation method of 5-bromo-2-chlorobenzoic acid in the present embodiment may further comprise the steps:
[0043] (1) Preparation of Compound III
[0044]Add 300mL of toluene, 40.0g of o-chlorobenzoic acid, 12mL of thionyl chloride, 0.5mL of DMF into a 500mL single-port reaction flask, stir at room temperature, slowly raise the temperature to 75°C, stop heating after four hours of heat preservation reaction, and cool down to room temperature; the reaction system is concentrated To dryness, add 100mL of dichloromethane; take another 500mL four-neck reaction flask, add 15g of 2-amino-2methyl-propanol, 100mL of dichloromethane, 25g of triethylamine, cool to -10°C, add dropwise The solution in the above-mentioned single-necked bottle was kept at a temperature lower than 0°C during the dropwise addition, and reacted at room temperature for 2 hours after the dropwise addition was completed;
[0045] Use 0.01moL of hydrochloric acid to adjust the pH to 5.5~6.5, separa...
Embodiment 3
[0050] The preparation method of 5-bromo-2-chlorobenzoic acid in the present embodiment may further comprise the steps:
[0051] (1) Preparation of Compound III
[0052] Add 300mL of toluene, 40.0g of o-chlorobenzoic acid, 50mL of thionyl chloride, and 0.5mL of DMF into a 500mL single-port reaction flask, stir at room temperature, slowly raise the temperature to 70°C, stop heating after keeping the reaction for eight hours, and cool down to room temperature; the reaction system is concentrated To dryness, add 100mL of dichloromethane; take another 500mL four-neck reaction flask, add 35g of 2-amino-2methyl-propanol, 100mL of dichloromethane, 50g of triethylamine, cool to -10°C, add dropwise The solution in the one-necked bottle mentioned above was kept at a temperature lower than 0°C during the dropping process, and reacted at room temperature for 3 hours after the dropping was completed;
[0053] Use 0.01moL of hydrochloric acid to adjust the pH to 5.5~6.5, separate layers, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com