Synthesis method and synthesis device of beta-nicotinamide mononucleotide
A single nucleotide and synthetic method technology, applied in the chemical field, can solve the problems of low reaction yield, unstable process, difficult purification of reaction intermediates and products, etc., and achieve the effect of reducing production costs and safe and reliable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
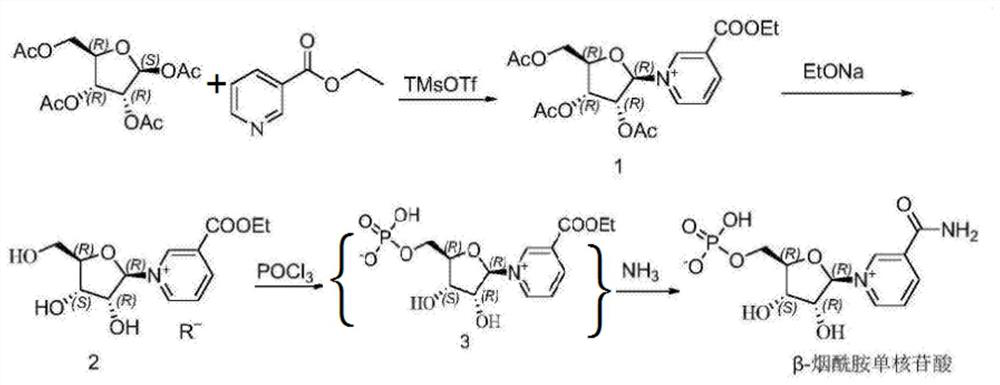
[0026] Please refer to figure 1 , the synthetic method of β-nicotinamide mononucleotide comprises the following steps:
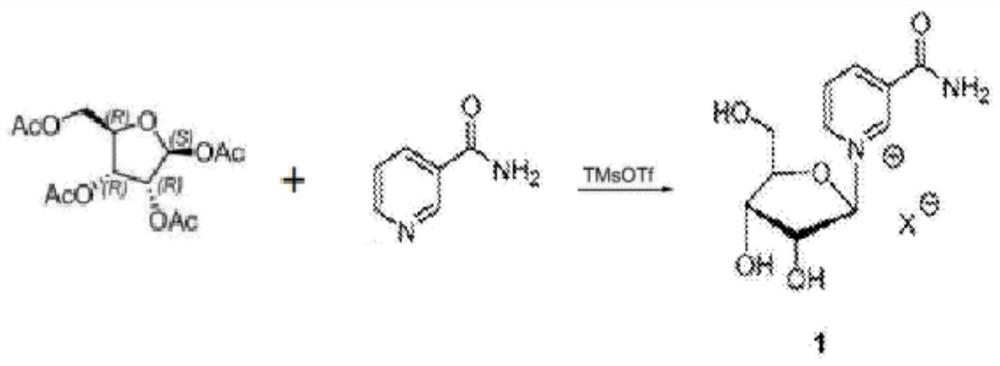
[0027] S1 condensation reaction, add 70 grams of ethyl nicotinate, 100 grams of tetraacetyl ribose and 1000 grams of chloroform into the reaction kettle, stir and dissolve at room temperature, then add 10 grams of trimethylsilyl trifluoromethanesulfonate in chloroform 100 grams of the solution, and then carry out condensation reaction at 50°C for 1 hour. After the reaction, cool down to room temperature, add 20 grams of ethanol to quench the reaction, remove the solvent under reduced pressure, and obtain 150 grams of triacetyl nucleoside containing nicotinic acid ethyl ester;
[0028] S2: Deacetylation, dissolve 150 grams of nicotinic acid ethyl triacetyl nucleoside in S1 in 1500 grams of ethanol, add dropwise an ethanol solution of 30 grams of sodium ethoxide at 0°C, react for 2 hours, and dropwise add Hydrochloric acid to neutrality, then add methyl tert-...
no. 2 example
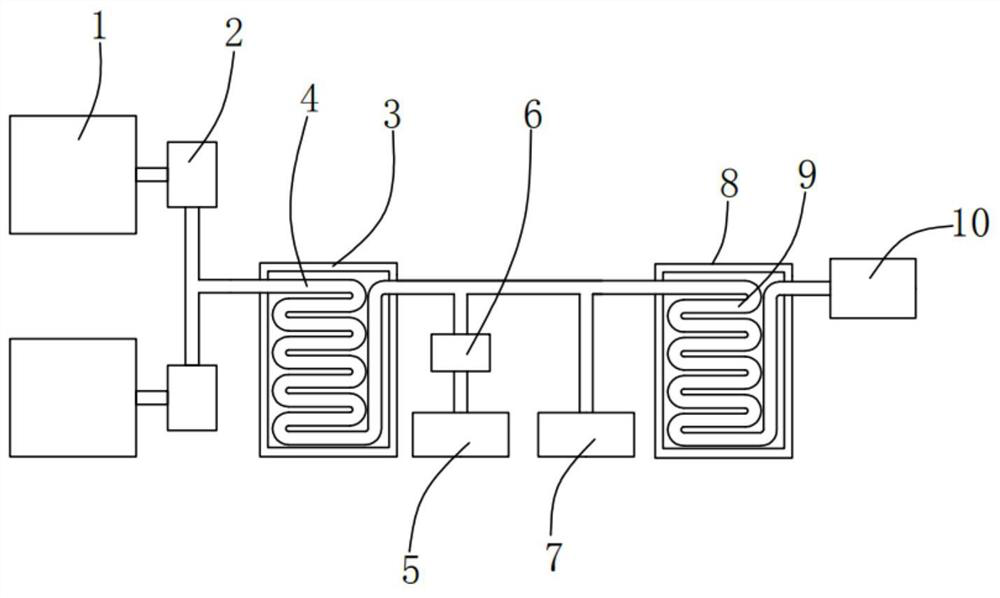
[0037] Please refer to figure 2 , a synthesis device for β-nicotinamide mononucleotide, which is applied to the synthesis method of β-nicotinamide mononucleotide, comprising: two mixing tanks 1, a first section tube reactor 3, purified water Tank 5, ammonia gas tank 7, second stage pipe reactor 8 and discharge tank 10; first metering pump 2, said first metering pump 2 is fixedly installed on said mixing tank 1, and said first metering The pump 2 is in communication with the mixing tank 1; the first reaction tube 4, the first reaction tube 4 is fixedly installed in the first section tube reactor 3, and the first reaction tube 4 is connected with the first metering The pump 2 is communicated with the ammonia gas tank 7; the second metering pump 6, the second metering pump 6 is fixedly installed on the pure water tank 5, and the second metering pump 6 is connected to the pure water tank 5 and The first reaction tube 4 communicates; the second reaction tube 9, the second reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com