Catalyst for synthesizing replacement ketone compounds and preparation method
A catalyst and compound technology, applied in the preparation of organic compounds, the preparation of carbon-based compounds, organic compounds/hydrides/coordination complex catalysts, etc. Wide scene, good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
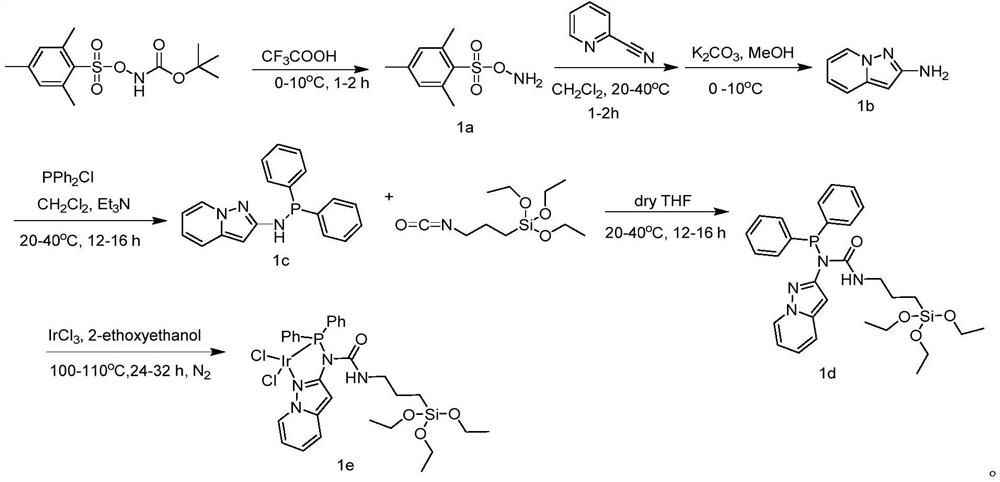
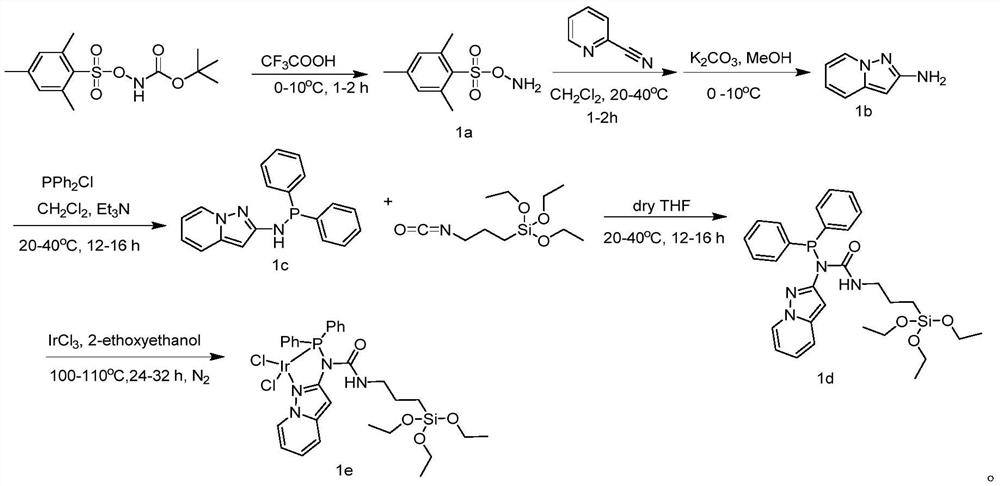
[0038] Embodiment 1: the synthesis of the iridium catalyst of nitrogen-containing phosphine ligand
[0039](1) Synthesis of 1a: At 0°C, 1 g of N-Boc-O-(isopropylideneacetonesulfonyl) hydroxylamine was placed in a 100 mL round bottom flask, and 10 mL of CF 3 COOH, the reaction was stirred at 0°C for 1 h; after the reaction was completed, ice water was slowly added to the mixture, and stirred again for 15 min, filtered with suction, the filter cake was collected, and washed with distilled water several times until pH = 7, and a colorless solid was obtained as the product 1a;
[0040] (2) Synthesis of 1b: 8mmol of 2-pyridineacetonitrile was dissolved in 20mL of dichloromethane, 12mmol of 1a was dissolved in 30mL of dichloromethane, and the dichloromethane of 1a was added dropwise to the 2-pyridineacetonitrile in dichloromethane solution. Chloromethane solution, react at room temperature for 1 hour after the dropwise addition, suction filter after the reaction, collect the filter...
Embodiment 2
[0046] Example 2: Catalyzing the reaction of benzyl alcohol and acetophenone to synthesize substituted ketones
[0047] Put 1mmol of benzyl alcohol and 1.1mmol of acetophenone into a 25mL reaction flask, then add 0.1mmol of the catalyst prepared in Example 1 and 0.05mmol of NaOH, use toluene as the reaction solvent, and place it in an oil bath at 120°C for 12h with magnetic force stirring After the reaction was completed and cooled to room temperature, distilled water was added, and extracted three times with ethyl acetate, the organic phase was collected, and the organic phase was rotary evaporated to dryness, and finally separated by column chromatography to obtain the product 1,3-diphenylpropane-1 - Ketone, the reaction yield was 88% by chromatographic analysis.
Embodiment 3
[0048] Example 3: Catalyzing the reaction of benzyl alcohol and 4-methoxyacetophenone to synthesize substituted ketones
[0049] Put 1mmol of benzyl alcohol and 1.1mmol of 4-methoxyacetophenone into a 25mL reaction flask, then add 0.1mmol of the catalyst prepared in Example 1 and 0.05mmol of NaOH, use toluene as the reaction solvent, and place in 120°C oil Magnetically stirred in the bath for 12 hours, after the reaction was completed and cooled to room temperature, distilled water was added, and extracted 3 times with ethyl acetate, the organic phase was collected, and the organic phase was rotary evaporated to dryness, and finally the product 1-(4 -Methoxyphenyl)-3-phenylpropan-1-one, the reaction yield was 89% by chromatographic analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com