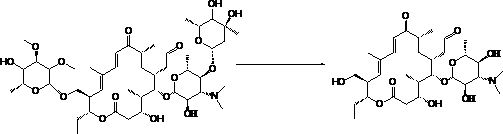
Method for quickly hydrolyzing 6-deoxy-D-allose in tylosin
A technology of tylosin and allose, which is applied in the field of synthesizing antibiotics for livestock and poultry, can solve the problems of difficulty in controlling the degree of side reactions, difficulty in controlling the process, long hydrolysis reaction time, etc., so as to reduce the content and cost of by-products. Low cost and the effect of improving the purity of intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Dissolve 30kg of tylosin in 900L of butyl acetate solution, cool down to 2-7°C, add 300L of purified water, adjust the pH of the aqueous phase to 2.5-5.5 with 0.1mol / L hydrochloric acid, and extract for 20 minutes. The organic phase was separated to obtain an aqueous solution of tylosin hydrochloride.
[0032] Dissolve 260g of polymer-dispersed carbon-based nano-solid acid containing sulfonic acid groups in 40L of ethylene glycol, and input it into the primary microreactor at a pressure of 0.08Mpa. Raise the temperature of the reactor to 65°C, circulate for 2 to 4 hours, stop the circulation, and discharge the ethylene glycol solution. The microreactor is rinsed with purified water to complete the immobilization of the polymer dispersed carbon-based nanometer solid acid on the microreaction.
[0033] The temperature of the microreactor loaded with polymer dispersed carbon-based nanometer solid acid was raised to 35° C., and the aqueous solution of tylosin hydrochloride...
Embodiment 2
[0038] Dissolve 150kg of tylosin in 5000L of ethyl acetate solution, cool down to 2-7°C, add 1800L of purified water, adjust the pH of the aqueous phase to 2.5-5.5 with 0.05mol / L hydrochloric acid, and extract for 30 minutes. The organic phase was separated to obtain an aqueous solution of tylosin hydrochloride.
[0039] Dissolve 280g of polymer dispersed carbon-based nanometer solid acid in 60L of ethylene glycol, and feed it into the primary microreactor at a pressure of 0.08Mpa. Raise the temperature of the reactor to 50°C, circulate for 3 to 7 hours, stop the circulation, and discharge the ethylene glycol solution. The microreactor is rinsed with purified water to complete the immobilization of the polymer dispersed carbon-based nanometer solid acid on the microreaction.
[0040]The temperature of the microreactor loaded with polymer dispersed carbon-based nanometer solid acid was raised to 37°C, and the aqueous solution of tylosin hydrochloride was injected at a pressure...
Embodiment 3
[0045] Dissolve 150kg of tylosin in 50L of methyl tert-butyl ether solution, cool down to 2-7°C, add 18L of purified water, and adjust the pH of the aqueous phase to 2.5-5.5 with 0.05mol / L hydrochloric acid. Extract for 30 minutes, and separate the organic phase to obtain an aqueous solution of tylosin hydrochloride.
[0046] 2.8g polymer dispersed carbon-based nanometer solid acid is dissolved in 600ml isopropanol, and is input in the primary microreactor with the pressure of 0.08Mpa. Raise the temperature of the reactor to 55° C., circulate for 5 hours, stop the circulation, and discharge the isopropanol solution. The microreactor is rinsed with purified water to complete the immobilization of the polymer dispersed carbon-based nanometer solid acid on the microreaction.
[0047] The temperature of the microreactor loaded with polymer dispersed carbon-based nanometer solid acid was raised to 43°C, and the aqueous solution of tylosin hydrochloride was injected at a pressure o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com