A kind of chiral catalyst (s)-dtp-cof and its preparation, recycling method and application
A -DTP-COF, chiral catalyst technology, applied in catalytic reactions, chemical instruments and methods, preparation of organic compounds, etc., to achieve the effect of improving utilization, simple method and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] A preparation method for chiral catalyst S-T-CCOF, the preparation steps are:
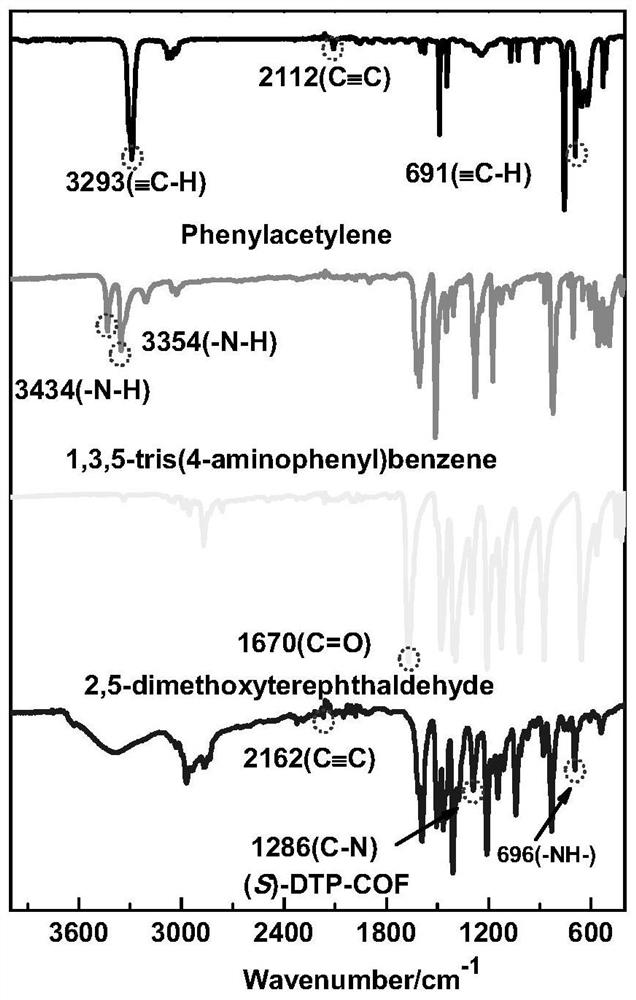
[0025] Place cuprous trifluoromethanesulfonate and (S,S)-2,6-bis(4-phenyl-2-oxazolin-2-yl)pyridine in a round-bottomed flask with chloroform as solvent, stirring dissolve. Add 2,5-dimethoxybenzene-1,4-dicarbaldehyde, 1,3,5-tris(4-aminophenyl)benzene, and phenylacetylene into a round-bottomed flask, and stir at room temperature for 48 hours. Finally, add acetic acid and stir for 24-48h to obtain (S)-DTP-COF.
[0026] In some embodiments, the cuprous trifluoromethanesulfonate, (S,S)-2,6-bis(4-phenyl-2-oxazolin-2-yl)pyridine, 2,5-bis The molar ratio of methoxybenzene-1,4-dicarbaldehyde, 1,3,5-tris(4-aminophenyl)benzene and phenylacetylene is 1:1:9:6:3.7 to improve reaction efficiency and yield .
[0027] Preferably, the amount of the chloroform solvent is 25mL to effectively dissolve cuprous trifluoromethanesulfonate, (S, S)-2,6-bis(4-phenyl-2-oxazolin-2-yl) pyridine.
[0028] Preferably,...
Embodiment 1
[0037] Embodiment 1: the synthesis of (S)-DTP-COF
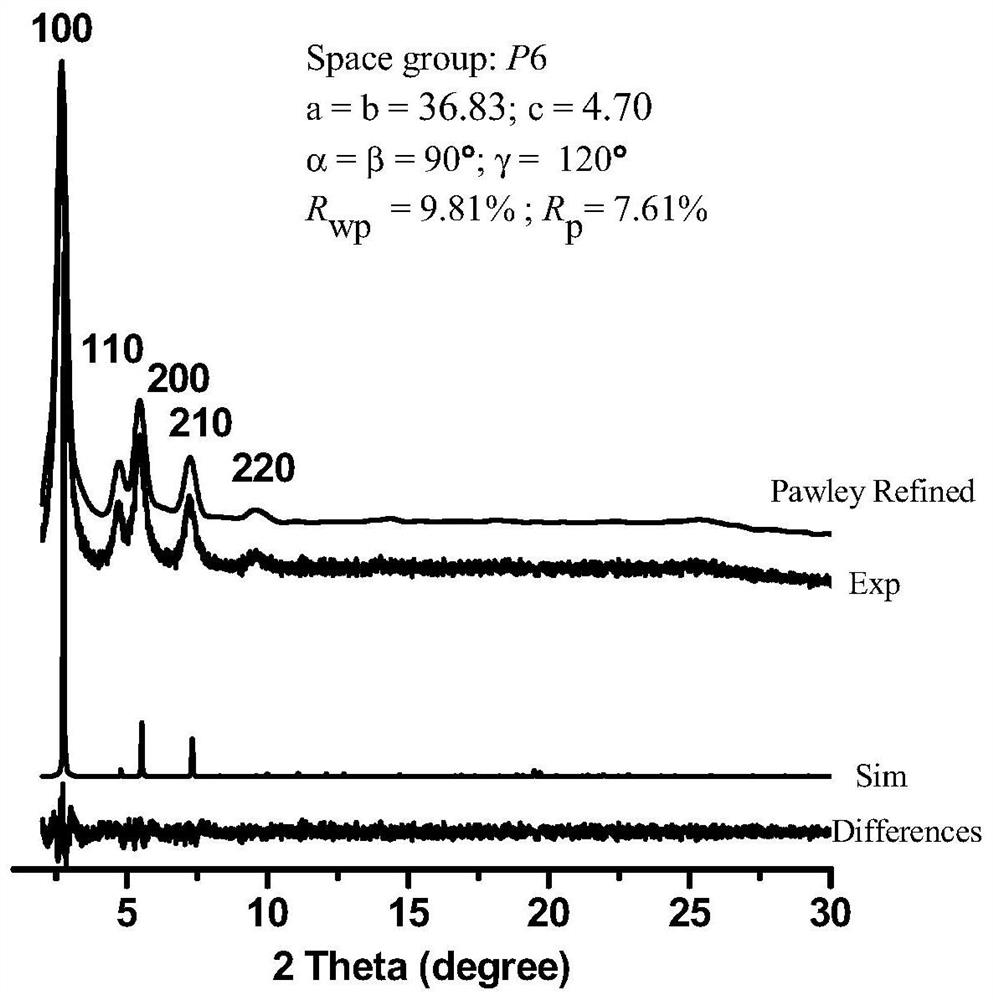
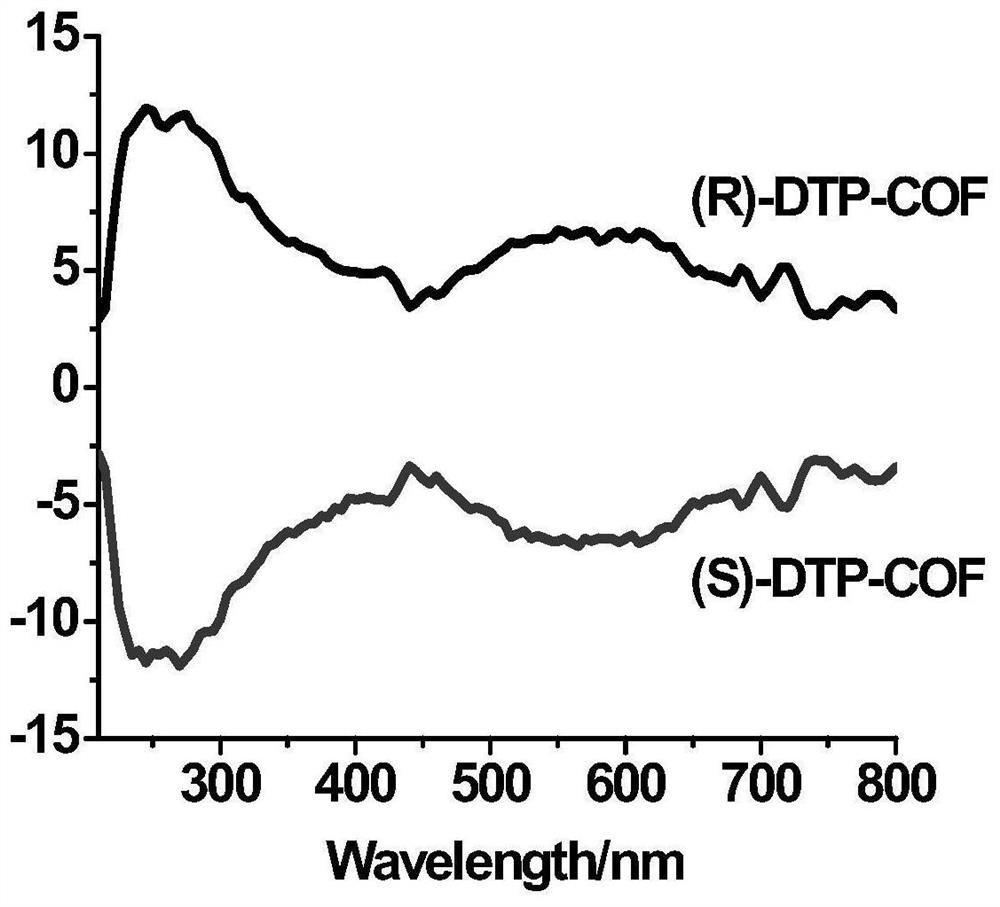
[0038] Put 12mg (0.02mmoL) of cuprous trifluoromethanesulfonate, 8mg (0.02mmoL) of (S, S)-2,6-bis(4-phenyl-2-oxazolin-2-yl)pyridine in 50ml In a round bottom flask, add 25ml of chloroform and stir to dissolve. 38.40mg (0.198mmoL) 2,5-dimethoxybenzene-1,4-dicarbaldehyde, 43mg (0.122mmoL) 1,3,5-three (4-aminophenyl) benzene, 90μl (0.819mmoL) Phenylacetylene was added to the round bottom flask and stirred at room temperature for 48h. Finally, 15μl of acetic acid was added and stirred for 24-48h. After the reaction was completed, centrifuged, washed 3 times with chloroform, and then washed 3 times with ethanol to obtain (S)-DTP-COF.
[0039] We characterized the polymer by IR, PXRD, CD spectra, the results are shown in figure 1 , 2 , 3.
[0040]
Embodiment 2
[0042] 1 ml (10 mmol) cyclohexanone, 344.4 mg (20% mol) pTsOH, EtOH (2 mL), 5 mg (3.3 mol%) (S)-DTP-COF were stirred at 25 °C for 10 minutes. Then (0.5 mmol) nitroalkene was added and the mixture was stirred at 25°C for 48 hours. After the reaction was finished, the reaction system was lowered to room temperature, and the catalyst was filtered out by centrifugation. After the solvent was removed by rotary evaporation, the product was purified through a silica gel column to obtain the corresponding product. Recover the catalyst, put it into the next reaction, directly separate and calculate the yield, the catalytic effect is as follows Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com