Method for preparing chiral duloxetine intermediate by aldehyde ketone reductase and asymmetric reduction
A technology of duloxetine and reductase, which is applied in the field of preparation of chiral duloxetine intermediates by aldehyde and ketone reductase and asymmetric reduction, which can solve the problems of low substrate tolerance, limited industrial application, long reaction time, etc. problems, to achieve the effect of convenient operation, simple equipment and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of recombinant engineered bacteria:
[0035] The DNA of Bacillus megaterium BM1-1 was extracted, and the kit used to extract the DNA was Ezup Column Bacteria Genomic DNA Extraction Kit.
[0036] DNA amplification was performed using the extracted Bacillus megaterium BM1-1 genome as a template to obtain the aldehyde and ketone reductase gene sequence shown in Appendix 1. The PCR reaction system is: 13 μL H 2 O, 1 μL upstream primer (primer sequence is F1: 5'GGAATTCCATATGATGCAATATCGAAAGCTTGGAAC3'), 1 μL downstream primer (primer sequence is R1: 5'CCGCTCGAGTTAATACAGTGAATTCACGGTATTC3'), 1 μL total DNA, 4 μL PrimeSTAR MaxPremix; PCR reaction conditions: 94 ° C pre-denaturation for 5 min , denatured at 94°C for 10s, annealed at 54°C for 10s, extended at 72°C for 6s, cycled 30 times, and extended at 72°C for 10 minutes; double-enzyme cut the aldoketone reductase gene and pET28a plasmid with NdeI and XhoI respectively, and ligated them with T4 DNA ligase Esche...
Embodiment 2
[0041] Step (1) to step (2) are the same as embodiment 1, and step (3) is as follows
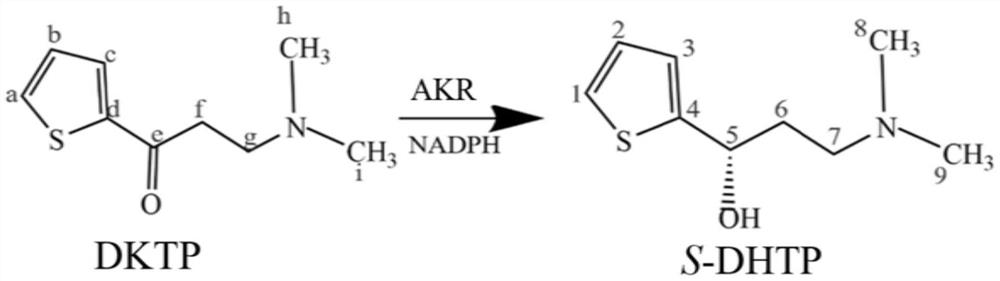
[0042] (3) Enzyme-catalyzed reaction: 5 mg of DKTP was dissolved in 5 mL of AKR3-2-9 enzyme solution, and NADPH was added (final concentration: 0.5 mM). Then, it was placed at 37° C. and reacted in a shaker at 200 r / min for 24 hours.
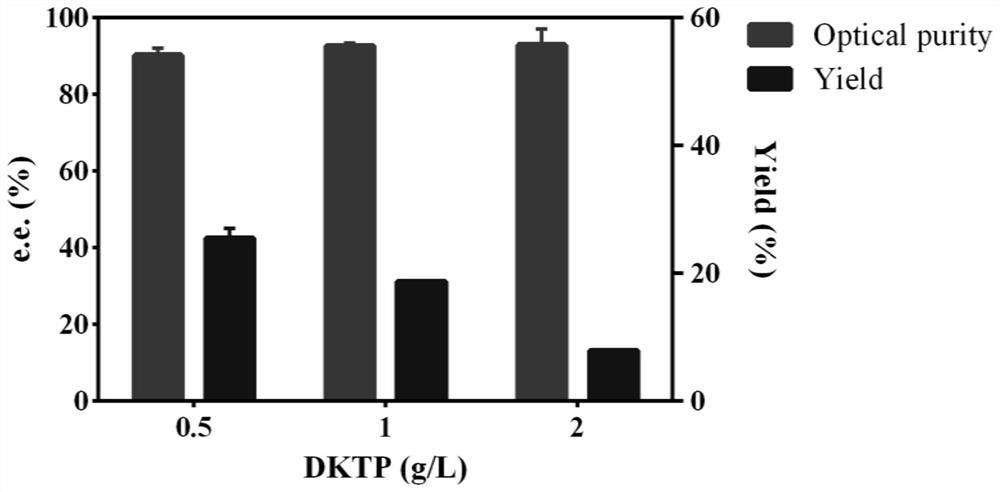
[0043] (4) Analysis and detection: analysis and detection: the concentration and enantiomeric excess value of the product S-DHTP are determined by high performance liquid chromatography. After the reaction was completed, the reacted sample was centrifuged at 4° C. and 10,000 r / min for 10 min, and the supernatant was taken. The enzyme catalyzes the substrate DKTP, and the product is quantitatively analyzed by high performance liquid chromatography. The yield of S-DHTP was 31%, and the optical purity was greater than 92%.
Embodiment 3
[0045] Step (1) to step (2) are the same as embodiment 1, and step (3) is as follows
[0046] (3) Enzyme-catalyzed reaction: 10 mg of DKTP was dissolved in 5 mL of AKR3-2-9 enzyme solution, and NADPH was added (final concentration: 0.5 mM). Then, it was placed at 37° C. and reacted in a shaker at 200 r / min for 24 hours.
[0047] (4) Analysis and detection: analysis and detection: the concentration and enantiomeric excess value of the product S-DHTP are determined by high performance liquid chromatography. After the reaction was completed, the reacted sample was centrifuged at 4° C. and 10,000 r / min for 10 min, and the supernatant was taken. The enzyme catalyzes the substrate DKTP, and the product is quantitatively analyzed by high performance liquid chromatography. The yield of S-DHTP was 13%, and the optical purity was greater than 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com