Preparation method of polysubstituted fluorine-containing six-membered nitrogen-containing heterocyclic methylamine with protecting groups
An extraction and extraction device technology, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of inconvenient vibration operation of the extraction device, difficulty in the implementation of the extraction process, and small volume of the extraction device.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
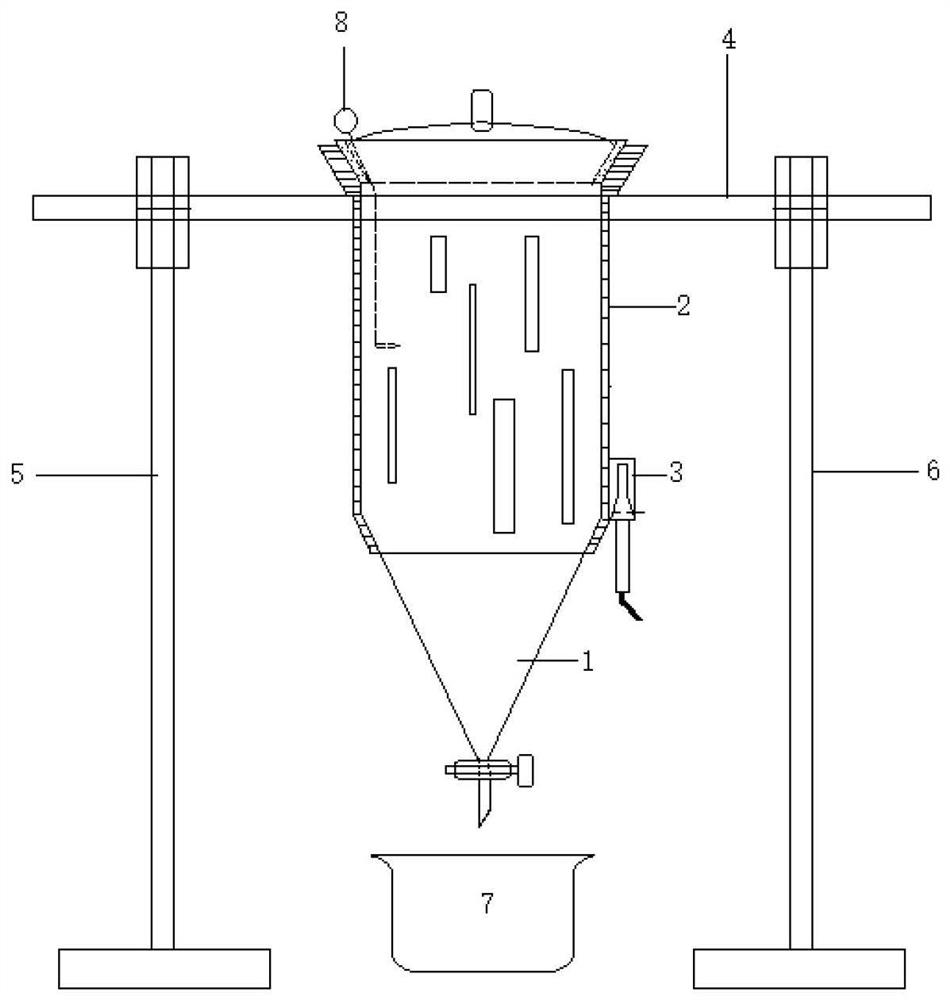
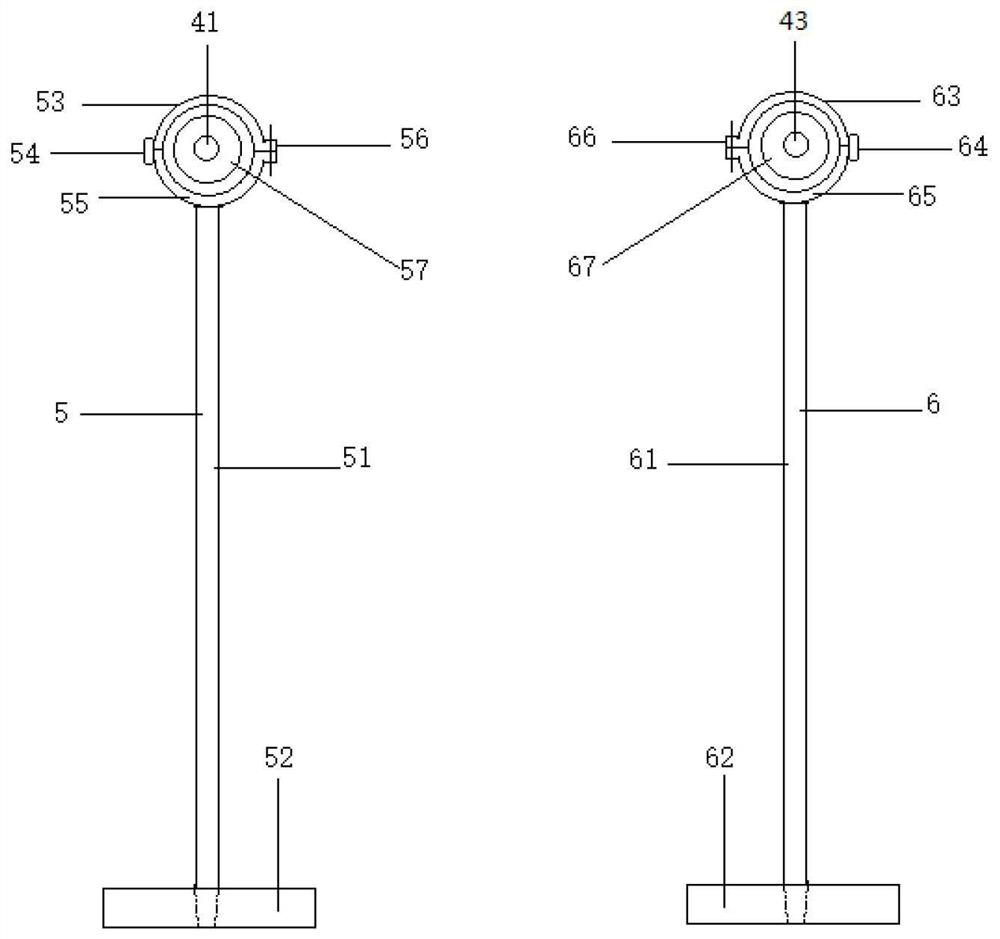
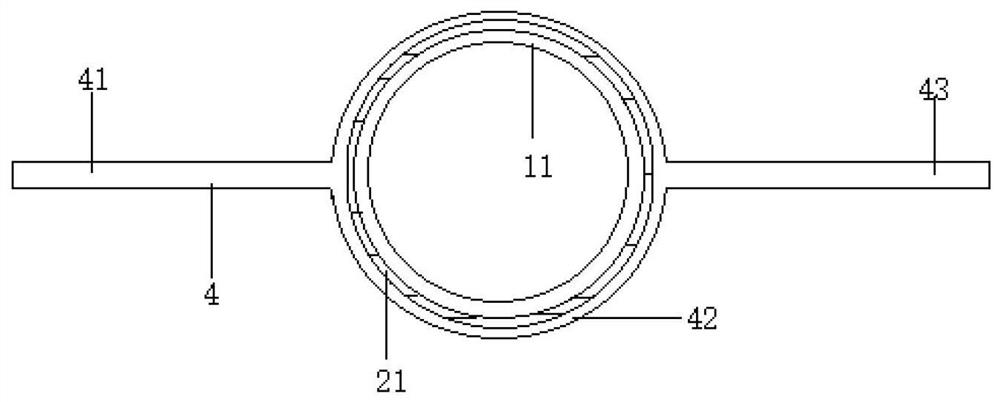
[0057] An ultrasonic extraction device is characterized in that it includes an extraction part 1 , a shell part 2 , an ultrasonic part 3 , a bar part 4 , a left support part 5 , a right support part 6 , a glassware 7 and an emulsion breaking component 8 . The extraction device can be divided into multiple specifications, for example, the volume of the extraction part can be 1L, 2L, 3L, 4L, 5L to meet different needs.
[0058]The extraction part 1 includes a cylindrical part 11 , an upper opening 12 , an upper cover 13 , a cone part 14 , a stopper set 15 and a liquid outlet 16 . The main part of the extraction part 1 is spliced from top to bottom by an upper opening 12, a cylindrical part 11, and a cone part 14. The cylindrical part 11 is a hollow cylinder, the upper opening 12 is large and the bottom is small, and the inner surface is a frosted part. 121, the outer edge of the upper cover 13 adapted to the frosted part 121 is a frosted surface, the upper center of the upper ...
Embodiment 2
[0067] A method for preparing a multi-substituted fluorine-containing nitrogen-containing heterocyclic methylamine with a protective group. Due to the large amount of extraction required, it is carried out using an ultrasonic extraction device as described above. It is characterized in that it includes the following step.
[0068] (1) Dissolve 9-11g of 2-hydroxy-5-bromopyridine-3-carboxylic acid in 105-115ml of methanol, stir until the system is colorless and transparent, heat up to 65°C and reflux, and start to dropwise add 13-14g of chlorinated Sulfoxide, 6-8min dropwise, the system gradually turns yellow and transparent, keep warm at 65°C until the reaction is monitored by HPLC, then lower the reaction mixture to room temperature, spin the reaction mixture to dryness and concentrate, add 50ml of ethyl acetate to dilute, and then Add 20ml of water to wash, and the solid precipitates out. The whole reaction mixture is suction-filtered, and the solid is beaten with 50ml of met...
Embodiment 3
[0079] A method for preparing a multi-substituted fluorine-containing nitrogen-containing heterocyclic methylamine with a protective group. Due to the large amount of extraction required, it is carried out using an ultrasonic extraction device as described above. It is characterized in that it includes the following step.
[0080] (1) Dissolve 11g of 2-hydroxy-5-bromopyridine-3-carboxylic acid in 115ml of methanol, stir until the system is colorless and transparent, heat up to 65°C and reflux, start to add 14g of thionyl chloride dropwise, and add dropwise for 8 minutes Complete, the system gradually becomes yellow and transparent, keep warm at 65°C until the reaction is monitored by HPLC, then the reaction mixture is lowered to room temperature, the reaction mixture is spin-dried and concentrated, diluted with 50ml of ethyl acetate, then washed with 20ml of water, and a solid is precipitated. The whole reaction mixture was filtered with suction, and the solid was beaten with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com