Preparation method of polysubstituted nitrogenous heterocyclic compound
A nitrogen heterocyclic compound, multi-substitution technology, applied in the field of organic synthesis, can solve the problems of unfriendly amine compounds, great environmental hazards, poor stability, etc., and achieve the effects of environmental friendliness, easy availability of raw materials, and avoidance of easy oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
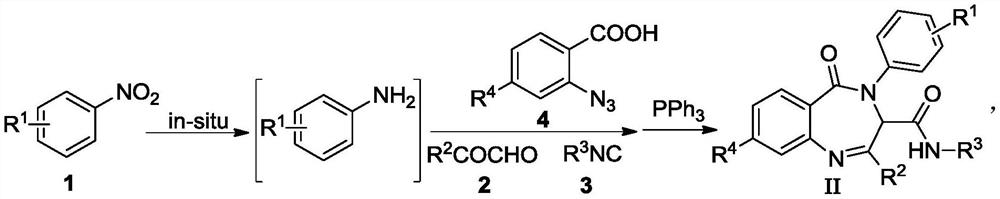
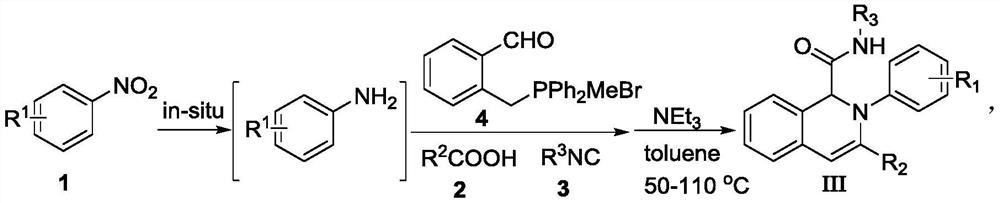
Image
Examples
Embodiment 1
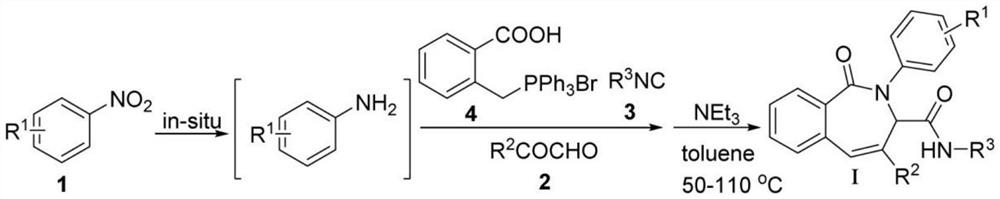
[0047] A method for preparing N-(tert-butyl)-4-(4-chlorophenyl)-1-oxo-2-phenyl-2,3-dihydro-1H-benzo[c]azepine-3-carboxamide, comprising the following experiments step:
[0048] Prepare a 25ml Schlenk reaction flask, adjust the temperature of the oil bath for heating to 60°C, add nitrobenzene 1 (0.123g, 1.0mmol, 1.0eqv.), methanol solvent (5ml), and Reducing agent B 2 (OH) 4 (0.448g, 5.0mmol, 5.0eqv.) was placed in an oil bath, heated and stirred to make it fully dissolved, and after the nitrobenzene was reduced to aniline as detected by TCL, the reaction was stopped. Lower the temperature of the oil bath to room temperature, and immediately add 2-((bromotriphenylphosphono)methyl)benzoic acid 2 (0.525g, 1.0mmol, 1.1eqv.), tert-butylisonitrile 3 (0.091g, 1.0mmol, 1.1 eqv.) and 2-(4-chlorophenyl)-2-oxoacetaldehyde 4 (0.185g, 1.0mmol, 1.1eqv.), reacted at room temperature for 24h, then added triethyl Amine (0.334g, 3.3mmol, 3.3eqv.) and toluene solvent (10ml) continued to reac...
Embodiment 2
[0052] A method for preparing N-(tert-butyl)-4-(4-chlorophenyl)-1-oxo-2-(p-tolyl)-2,3-dihydro-1H-benzo[c]azepine-3-carboxamide , including the following experimental steps:
[0053] Prepare a 25ml Schlenk reaction flask, adjust the temperature of the oil bath used for heating to 60°C, add 4-methylnitrobenzene 1 (0.137g, 1.0mmol, 1.0eqv.) and methanol solvent ( 5ml), and reducing agent B 2 (OH) 4 (0.448g, 5.0mmol, 5.0eqv.) was placed in an oil bath, heated and stirred to make it fully dissolved, and after the nitrobenzene was reduced to aniline as detected by TCL, the reaction was stopped. Lower the temperature of the oil bath to normal temperature, and immediately add 2-((bromotriphenylphosphono)methyl)benzoic acid 2 (0.525g, 1.0mmol, 1.1eqv.), tert-butylisonitrile 3 (0.091g, 1.0mmol, 1.1eqv.) and 2-(4-chlorophenyl)-2-oxoacetaldehyde 4 (0.185g, 1.0mmol, 1.1eqv.), reacted at room temperature for 24h, then added triethyl Amine (0.334g, 3.3mmol, 3.3eqv.) and toluene solvent (...
Embodiment 3
[0057] A method for preparing N-(tert-butyl)-4-(4-chlorophenyl)-1-oxo-2-(o-tolyl)-2,3-dihydro-1H-benzo[c]azepine-3-carboxamide , including the following experimental steps:
[0058] Prepare a 25ml Schlenk reaction flask, adjust the temperature of the oil bath used for heating to 60°C, add 2-methylnitrobenzene 1 (0.137g, 1.0mmol, 1.0eqv.) and methanol solvent ( 5ml), and reducing agent B 2 (OH) 4 (0.448g, 5.0mmol, 5.0eqv.) was placed in an oil bath, heated and stirred to make it fully dissolved, and after the nitrobenzene was reduced to aniline as detected by TCL, the reaction was stopped. Lower the temperature of the oil bath to normal temperature, and immediately add 2-((bromotriphenylphosphono)methyl)benzoic acid 2 (0.525g, 1.0mmol, 1.1eqv.), tert-butylisonitrile 3 (0.091g, 1.0mmol, 1.1eqv.) and 2-(4-chlorophenyl)-2-oxoacetaldehyde 4 (0.185g, 1.0mmol, 1.1eqv.), reacted at room temperature for 24h, then added triethyl Amine (0.334g, 3.3mmol, 3.3eqv.) and toluene solvent (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com