Preparation method of methylamine
A technology for methyl amine and amine methylation, applied in the field of preparing methyl amine, can solve the problems of complex methyl amine production links, unstable organic catalysts, harsh reaction conditions, etc., to simplify the post-processing process and avoid device performance. Reduced, silicophilic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
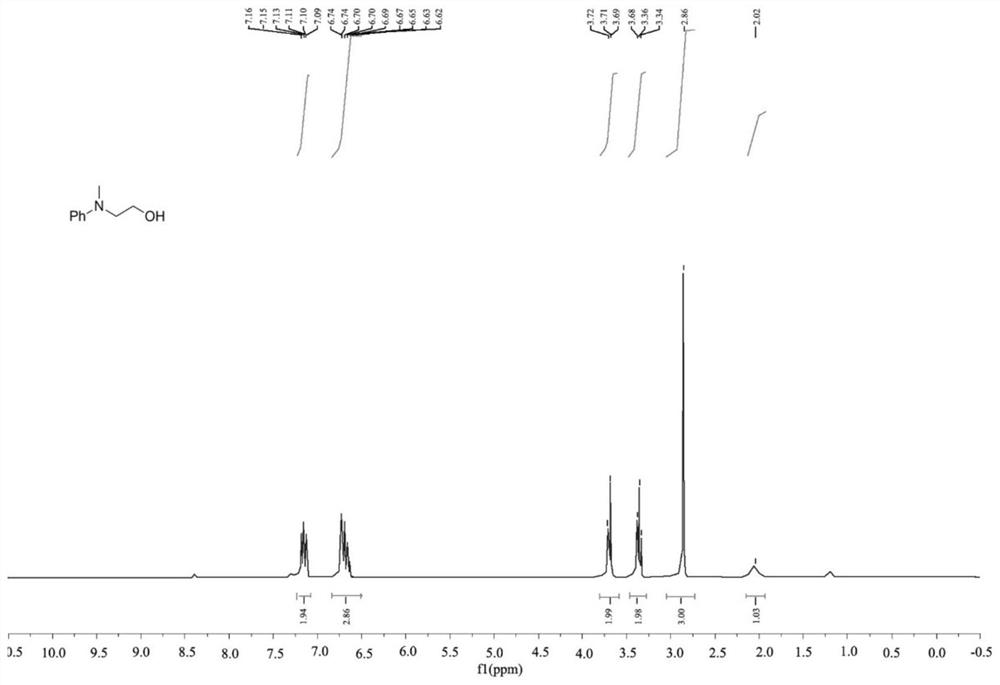
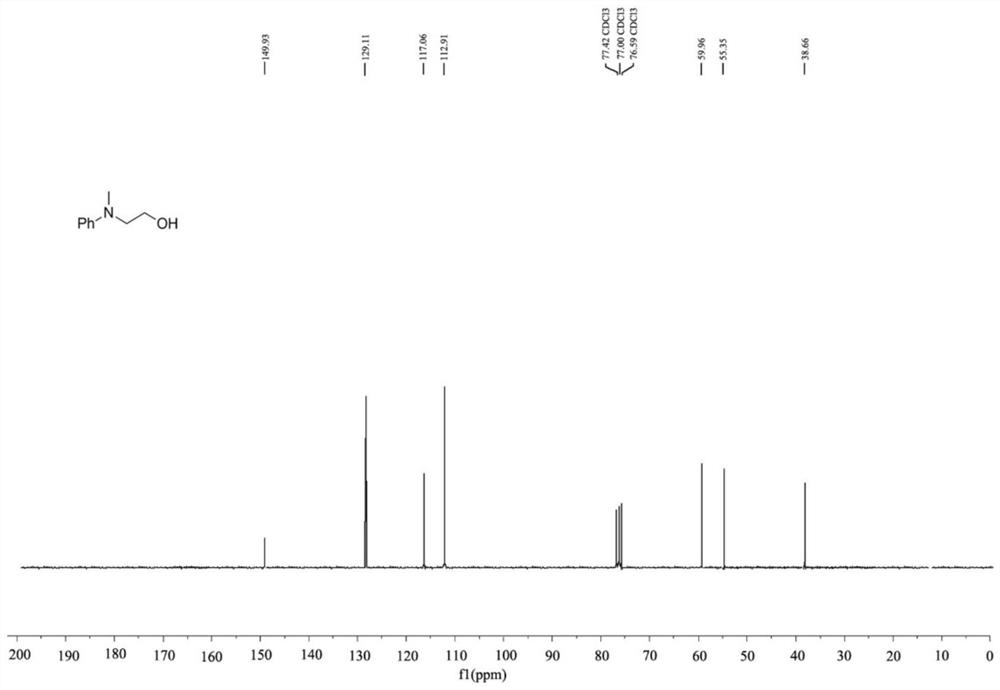
[0041] This embodiment provides a method for preparing methylamine, wherein the catalyst used is dodecyldimethylamine caprolactone, the methylamine is 2-(methyl(phenyl)amino)ethanol, and methyl The reaction scheme of the amine is as follows:
[0042]
[0043] The preparation method of 2-(methyl (phenyl) amino) ethanol comprises the following steps:
[0044] Take a strictly dry Shrek reaction tube (Schlenk tube), connect a balloon filled with carbon dioxide to the Schlenk tube through the side tube, and wash 3 times, add dodecyldimethylamine caprolactone (5mol % relative to amine), 2-(phenylamino)ethanol (0.25 mmol), polymethylpolysiloxane PMHS (1 mmol) and CH 3 CN (2mL), Schlenk tube heated at 70°C for 12h, cooled to room temperature, released CO in the balloon 2 , EtOAc (10mL) was added to the mixture to quench the reductive reaction, and CH 2 Cl 2 Extract three times, concentrate the organic layer in anhydrous Na 2 SO 4 Drying on , evaporated in vacuo and column chr...
Embodiment 2
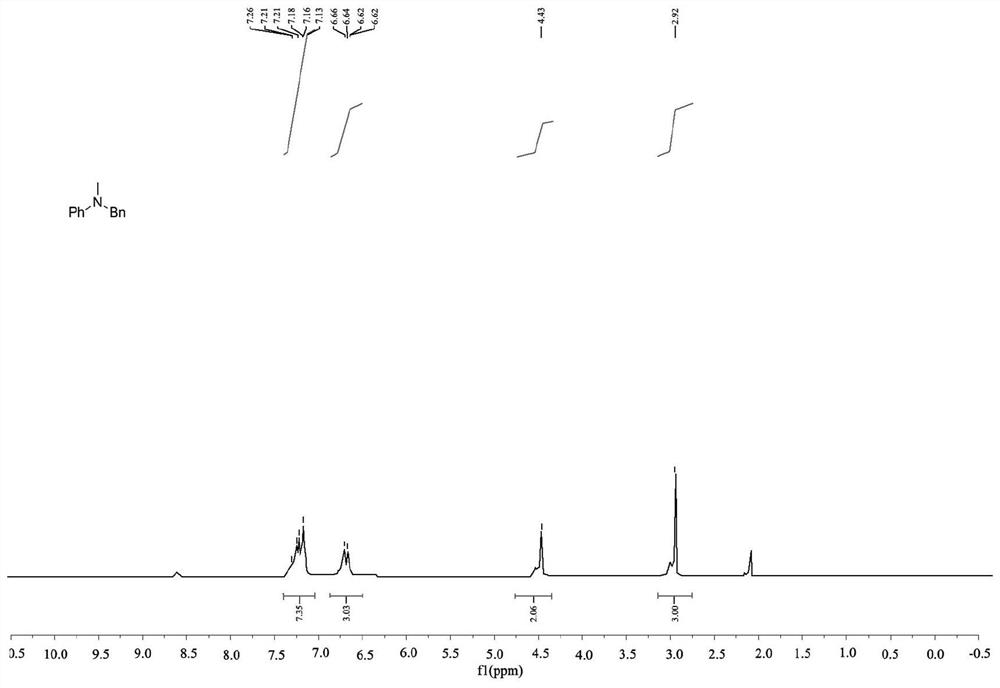
[0048] This embodiment provides a method for preparing methylamine, wherein the catalyst used is dodecyldimethylamine caprolactone, and the methylamine is N-benzyl-N-methylaniline. The reaction scheme is as follows:
[0049]
[0050] The preparation method of N-benzyl-N-methylaniline comprises the following steps:
[0051] Take a strictly dry Shrek reaction tube (Schlenk tube), connect a balloon filled with carbon dioxide to the Schlenk tube through the side tube, and wash 3 times, add dodecyldimethylamine caprolactone (5mol % relative to amine), N-benzylaniline (0.25 mmol), polymethylpolysiloxane PMHS (1 mmol) and CH 3 CN (2mL), Schlenk tube heated at 50°C for 10h, cooled to room temperature, released CO in the balloon 2 , EtOAc (10mL) was added to the mixture to quench the reductive reaction, and CH 2 Cl 2 Extract three times, concentrate the organic layer in anhydrous Na 2 SO 4 Drying on , evaporated in vacuo and column chromatography on silica gel with petroleum e...
Embodiment 3
[0055] This example provides a method for preparing methylamine, wherein the catalyst used is dodecyldimethylamine caprolactone, and the methylamine is N,N-dimethyl-[1,1'-diphenyl Base]-2-amine, the reaction scheme of preparing methylamine is as follows:
[0056]
[0057] The preparation method of N,N-dimethyl-[1,1'-diphenyl]-2-amine comprises the following steps:
[0058] Take a strictly dry Shrek reaction tube (Schlenk tube), connect a balloon filled with carbon dioxide to the Schlenk tube through the side tube, and wash 3 times, add dodecyldimethylamine caprolactone (5mol % relative to amine), N-methyl-[1,1'-diphenyl]-2-amine (0.25mmol), polymethylpolysiloxane PMHS (1.2mmol) and CH 3 CN (2mL), Schlenk tube heated at 60°C for 6h, cooled to room temperature, release the CO in the balloon 2 , EtOAc (10mL) was added to the mixture to quench the reductive reaction, and CH 2 Cl 2 Extract three times, concentrate the organic layer in anhydrous Na 2 SO 4 Drying on , evapor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com