Method for separating ertugliflozin and isomers thereof
A separation method and isomer technology, applied in the field of pharmaceutical analysis, can solve the problems of no separation method, etc., and achieve the effect of low equipment requirements, strong practicability, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The residence time analysis of the isomers of Egliflozin specifically includes the following steps:
[0046] (1) Preparation of diluent
[0047] Mix acetonitrile and distilled water at a volume ratio of 1:1 to obtain a dilution;
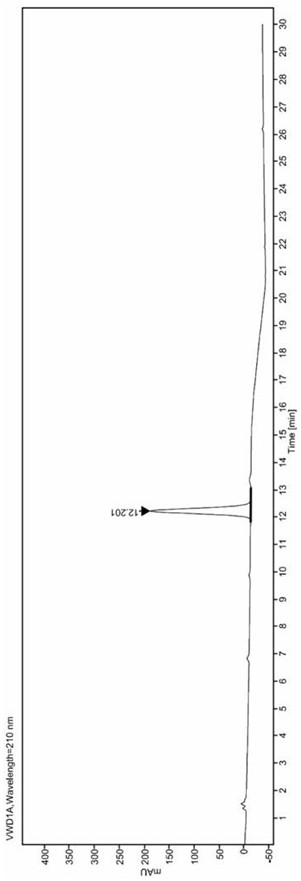
[0048] (2), preparation of Epagliflozin isomer solution
[0049] Accurately weigh 25.0mg of Epagliflozin isomers, in a 50mL volumetric flask, dilute to the 50mL mark with the diluent obtained in step (1), mix well to obtain the Epagliflozin Isomers solution;
[0050] (3), adopt reversed-phase high performance liquid chromatography, carry out chromatographic analysis to the diluent of above-mentioned step (1) gained and the epagliflozin isomer solution of step (2) gained, chromatographic analysis condition is as follows:
[0051] The chromatographic column is Agilent proshell 120 EC-C18 4.6×150mm, 4μm;
[0052] The temperature of the chromatographic column is controlled at 35°C;
[0053] The filler used in the chromatographic column is octa...
Embodiment 2
[0061] A kind of Epagliflozin and the analysis of the residence time and resolution of Epagliflozin isomers in different chromatographic columns, specifically comprise the steps:
[0062] (1) Preparation of diluent
[0063] Mix acetonitrile and distilled water at a volume ratio of 1:1 to obtain a dilution;
[0064] (2), the preparation of Epagliflozin solution
[0065] Accurately weigh 25.0mg of Epagliflozin, in a 50mL volumetric flask, dilute to the 50mL mark with the diluent obtained in step (1), mix well to obtain the Epagliflozin solution;
[0066] (3), preparation of Epagliflozin isomer solution
[0067] Accurately weigh 25.0mg of Epagliflozin isomers, in a 50mL volumetric flask, dilute to the 50mL mark with the diluent obtained in step (1), mix well to obtain the Epagliflozin Isomers solution;
[0068] (4) adopt reverse-phase high-performance liquid chromatography, carry out chromatographic analysis respectively to the epagliflozin solution of step (2) gained and the ...
Embodiment 3
[0073] A mixture of Epagliflozin and its isomers, the analysis of residence time and resolution in different mobile phase ratios, specifically comprises the following steps:
[0074] (1) Preparation of diluent
[0075] Mix acetonitrile and distilled water at a volume ratio of 1:1 to obtain a dilution;
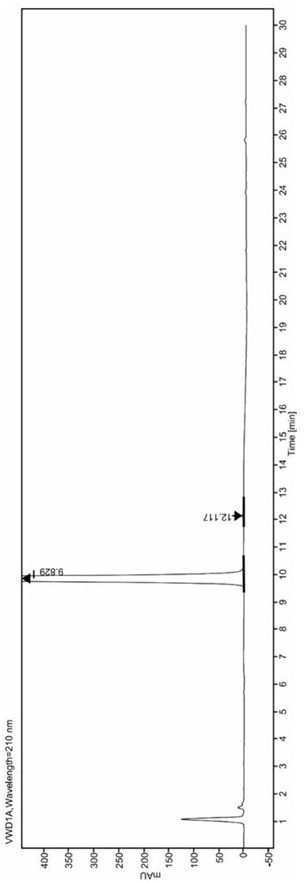
[0076] (2), the preparation of the mixed solution of Epagliflozin and Epagliflozin isomers
[0077] Accurately weigh 25.0mg of Epagliflozin, 0.5mg of Epagliflozin isomers in a 50mL volumetric flask, dilute to the 50mL scale with the diluent obtained in step (1), mix well to obtain Epagliflozin and Epagliflozin A mixed solution in which the net isomers are mixed;
[0078] (3), adopt reverse-phase high-performance liquid chromatography, carry out chromatographic analysis to the mixed solution that the Epagliflozin of step (2) gained and Epagliflozin isomers mix, chromatographic analysis conditions, chromatographic analysis conditions are as follows:
[0079] Chromatographic se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com