A kind of in situ injection jelly and its preparation method and application
A technology of in situ injection and jelly, which is applied in drug delivery, medical formulation, surgery, etc., can solve the problems of insufficient bulge height, large injection volume, high osmotic pressure, etc., and achieve optimal maintenance time and height, hemostasis, wound healing, The effect of promoting wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
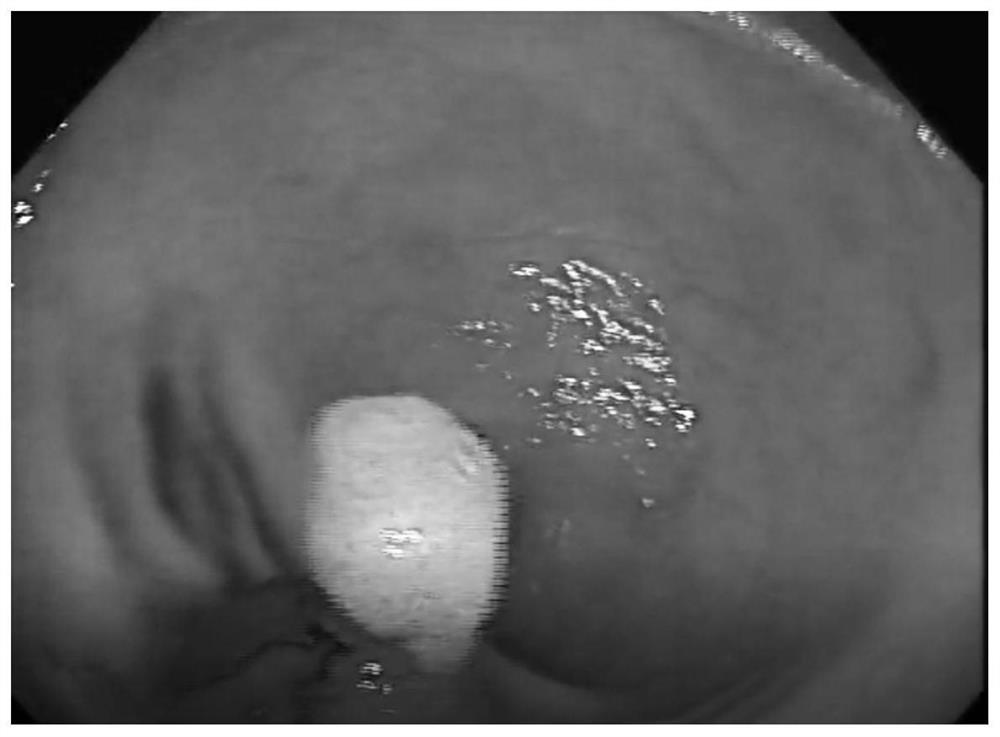
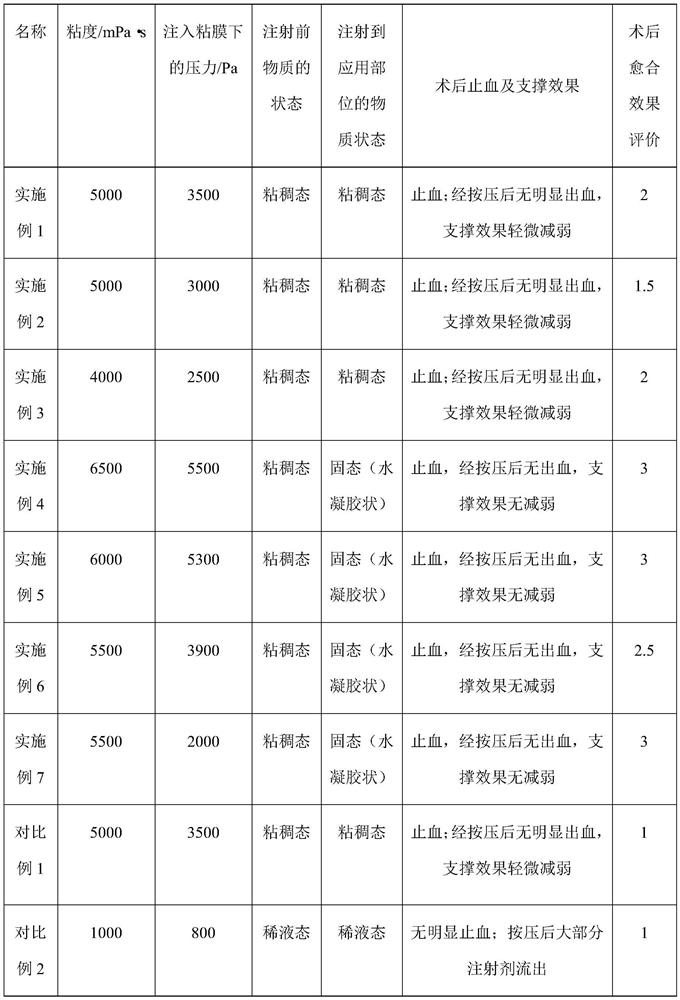
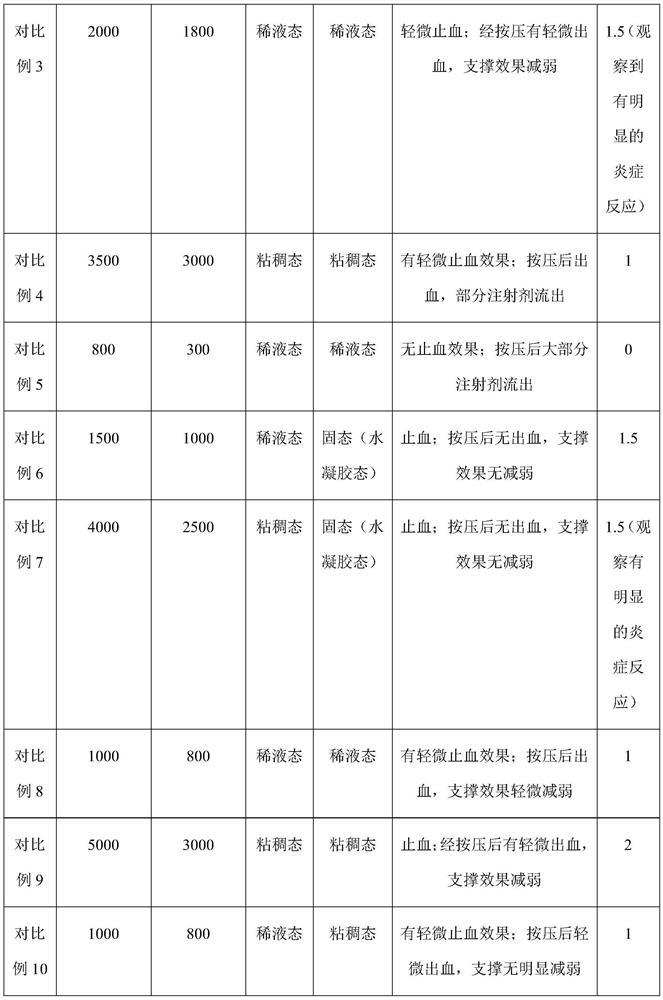
[0058] Weigh 1 g of sodium starch glycolate (molecular weight is about 120,000 Da), 0.2 g of lysine for physical mixing, and then dissolve in 20 mL of glucose injection to obtain in situ injection jelly.
Embodiment 2
[0060] Weigh 1g sodium starch glycolate (molecular weight is about 80000Da), mix physically with 0.005g glycine, and then dissolve in 10mL phosphate buffer (0.1M, pH=7.4); the in situ injection jelly is obtained.
Embodiment 3
[0062] Weigh 1 g of carmellose sodium (molecular weight is about 300,000 Da), 0.1 g of oligoglutamic acid (molecular weight of about 1200 Da) and 0.001 g of brilliant blue are physically mixed, and then dissolved in 15 mL of normal saline injection; In situ injection jelly was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com