Electroluminescent device based on chiral thermal activation delayed fluorescence material and preparation method thereof
An electroluminescent device, thermal activation delay technology, applied in the direction of luminescent materials, electro-solid devices, chemical instruments and methods, etc. problem, to achieve the effect of high yield, few preparation steps and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The reaction formula is as follows:
[0052]
[0053] The reaction is as follows:
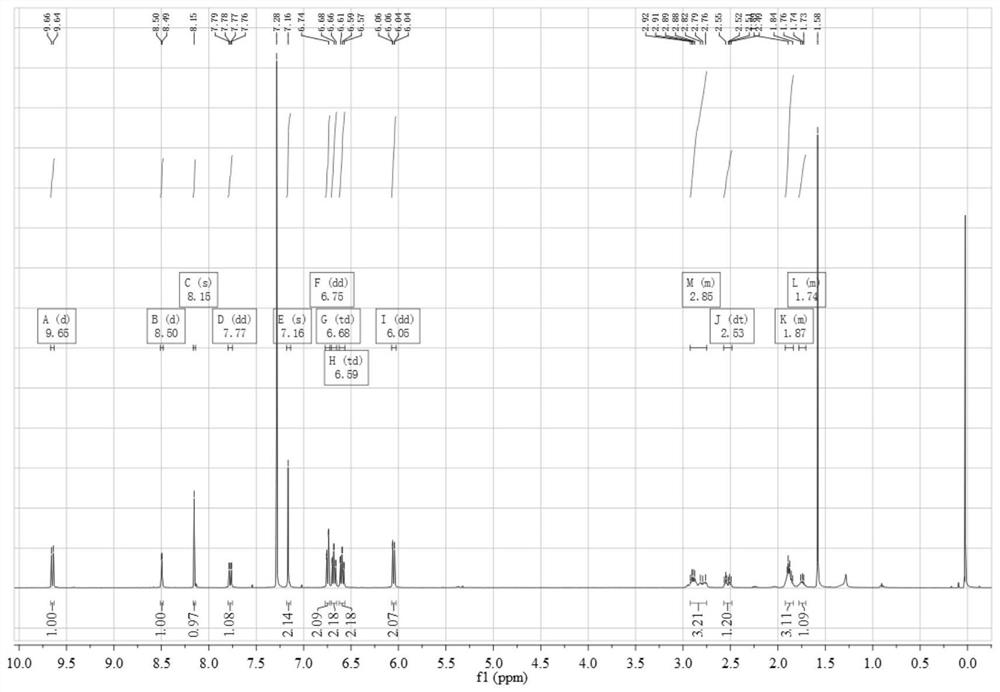
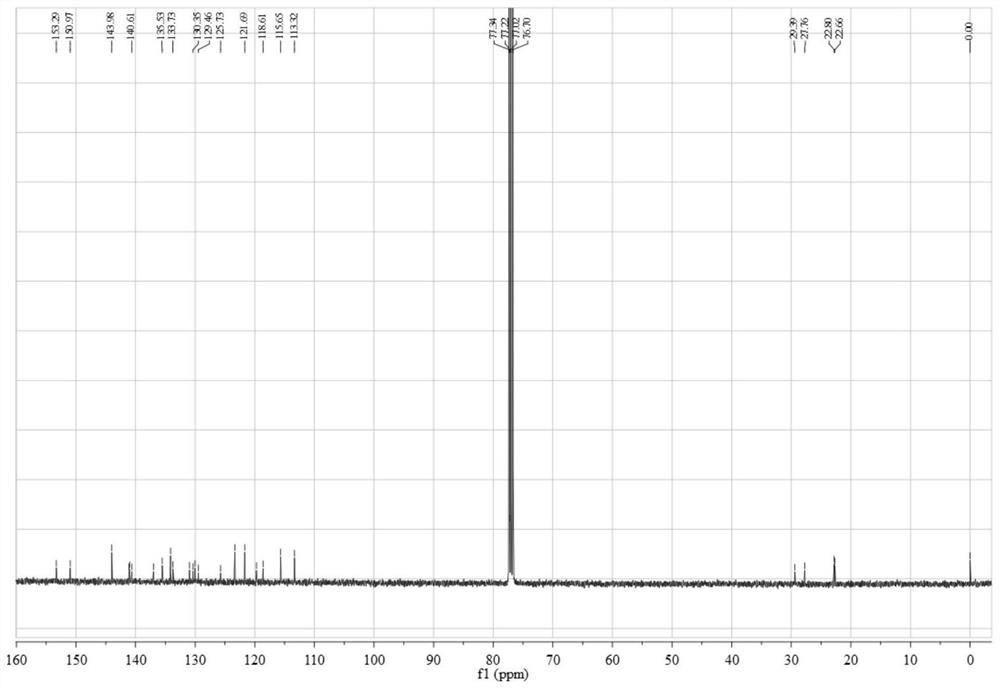
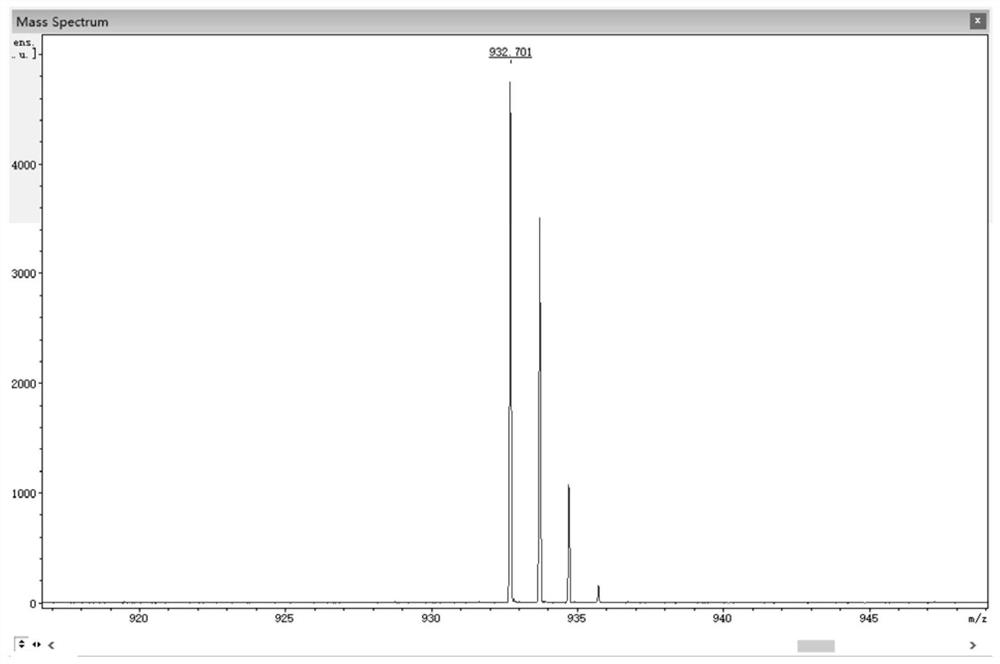
[0054] Add 0.45 g (3.12 mmol) of 4,5-difluorobenzene-1,2-diamine and 1.12 g (3.06 mmol) of 3,6-dibromo-9,10-phenanthrenequinone into a 150 mL three-necked flask, and then add 100 mL of absolute ethanol as a solvent, stirred under the protection of nitrogen, reacted at 80 °C for 2 hours, then filtered the reaction solution, and recrystallized the filter cake with absolute ethanol to obtain a light yellow solid 3,6-dibromo-11,12-di Fluorodibenzo[a,c]phenazine in 95% yield.
[0055] 0.50 g (1.05 mmol) 3,6-dibromo-11,12-difluorodibenzo[a,c]phenazine, 0.40 g (2.18 mmol) 10H-phenoxazine, 0.41 g (4.27 mmol) sodium tert-butoxide, 0.016 g (0.055 mmol) tri-tert-butylphosphine tetrafluoroborate, 0.05 g (0.055 mmol) tris(dibenzylideneacetone) dipalladium (0), then add 50 mL toluene As a solvent, the reaction was heated at 100 °C under the protection of nitrogen; after the reaction was complete...
Embodiment 2
[0063] The reaction formula is as follows:
[0064]
[0065] The reaction is as follows:
[0066] 0.45 g (3.12 mmol) 4,5-difluorobenzene-1,2-diamine and 1.12 g (3.04 mmol) 1,2-bis(4-bromophenyl)ethane-1 were added to a 150 mL three-neck flask, 2-diketone, then add 100 mL of absolute ethanol as a solvent, stir under the protection of nitrogen, react at 80 °C for 2 hours, then filter the reaction solution, and recrystallize the filter cake with absolute ethanol to obtain a light yellow solid 2,3- Bis(4-bromophenyl)-6,7-difluoroquinoxaline, yield 95%.
[0067]1.00 g (2.10 mmol) 2,3-bis(4-bromophenyl)-6,7-difluoroquinoxaline, 0.80 g (4.36 mmol) 10H-phenoxazine, 1.00 g (10.4 mmol) sodium tert-butoxide, 0.032 g (0.11 mmol) tri-tert-butylphosphine tetrafluoroborate, 0.10 g (0.11 mmol) tris(dibenzylideneacetone) dipalladium (0), and then add 50 mL toluene for Solvent, heat reaction at 100 ℃ under the protection of nitrogen; after the reaction is completed, extract with 100 mL of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dissymmetry factor | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
| dissymmetry factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com