Tetrandrine liposome, preparation method thereof and medical mask containing tetrandrine liposome
A technology of tetrandrine and alkali lipids, which is applied to chemicals, applications, and protective clothing for biological control. It can solve the problems of low encapsulation efficiency and drug loading, and achieve high drug loading and encapsulation efficiency. High, enhanced protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The present embodiment discloses the preparation method of the tetrandrine liposome of the present invention, specifically as follows:
[0055]Weigh soybean lecithin (0.36g), cholesterol (0.036g), Tween-80 (0.072g), and tetrandrine (0.03g) respectively, add absolute ethanol (15mL) to fully dissolve, remove the solvent by evaporation under reduced pressure, in A uniform film is formed on the bottom of the bottle. Add pH 6.5 phosphate buffer (30 mL), and hydrate at 30° C. for 15 minutes to obtain liposome colostrum. Then through 500bar high-pressure homogenization three times, each homogenization time is 32s, namely the tetrandrine liposome solution. The measured particle size is 327.2nm (PDI=0.558), the potential is 3.93mV, the encapsulation efficiency is 83.13%, and the drug loading is 6.17%.
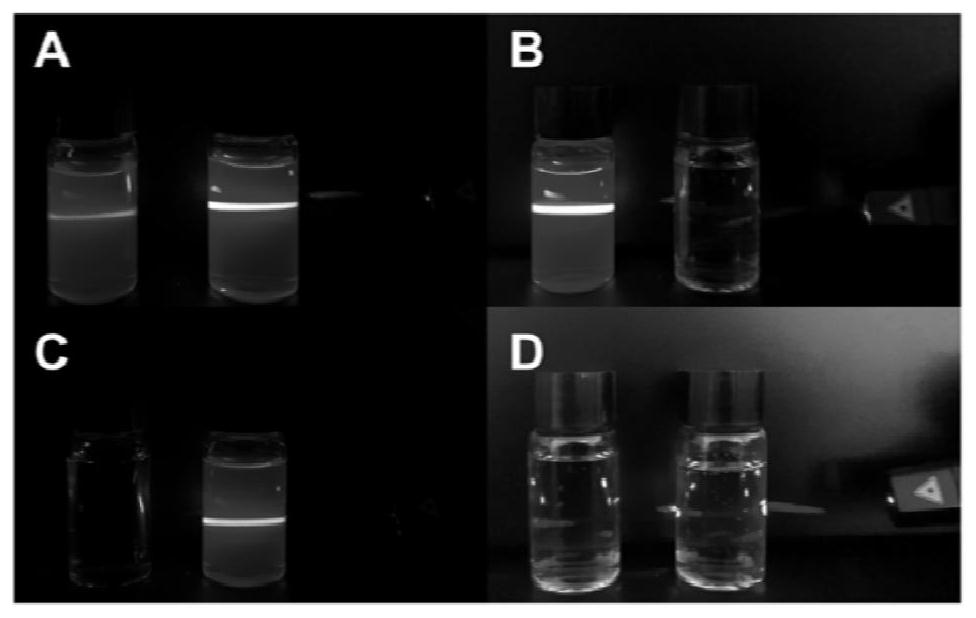
[0056] The tetrandrine liposomes prepared in this example have Tyndall effect and nanometer size. as attached figure 1 As shown, there will be a light path when the tetrandri...
Embodiment 2
[0059] The present embodiment discloses the preparation method of the tetrandrine liposome of the present invention, specifically as follows:
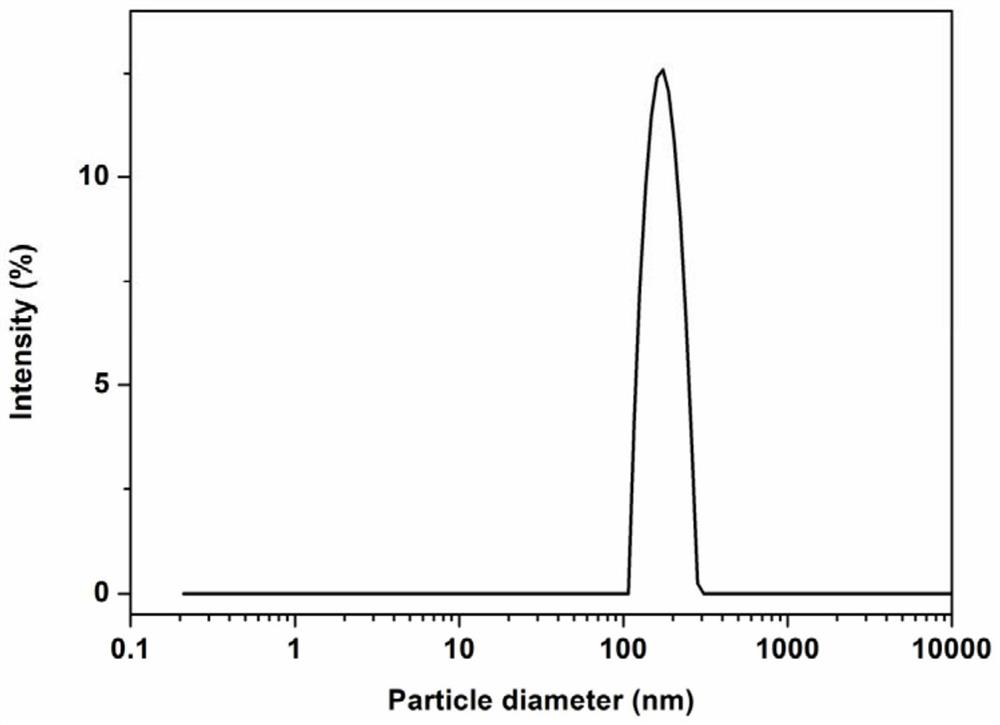
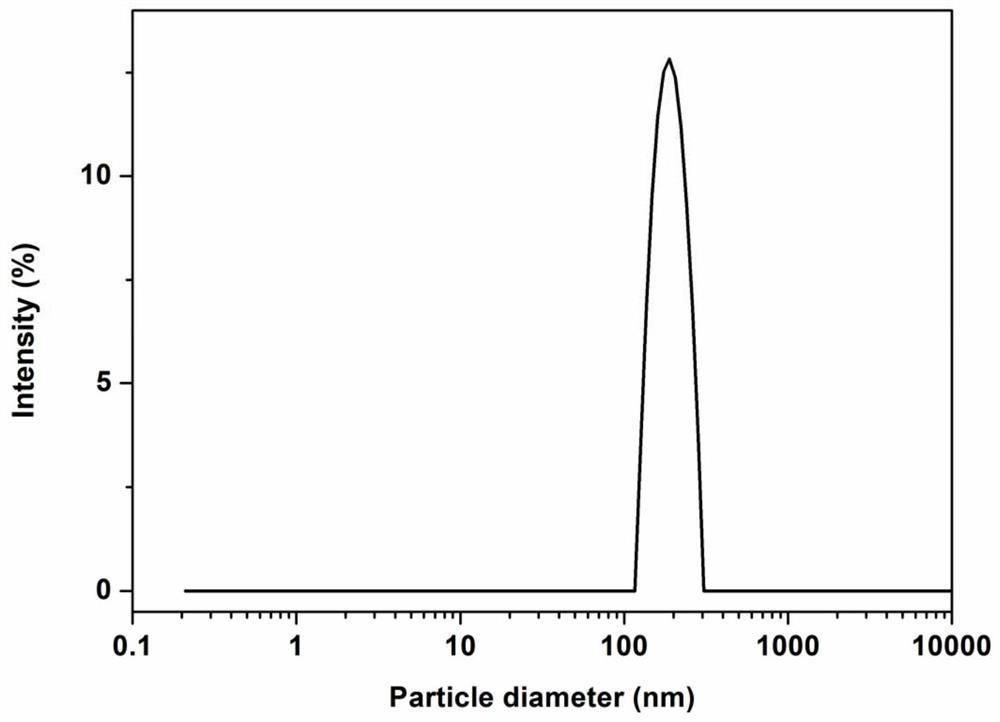
[0060] Weigh soybean lecithin (0.36g), oleic acid (0.154g), cholesterol (0.036g), Tween-80 (0.072g), tetrandrine (0.03g), add absolute ethanol (30mL) to fully dissolve, The solvent was removed by evaporation under reduced pressure, and a uniform film was formed on the bottom of the bottle. Add pH 6.5 phosphate buffer (30 mL), and hydrate at 30° C. for 15 minutes to obtain liposome colostrum. Then through 500bar high-pressure homogenization three times, each homogenization time is 32s, namely the tetrandrine liposome solution. The measured particle size is 119.72nm (PDI=0.225), the potential is -23.3mV, the encapsulation efficiency is 98.79%, and the drug loading is 4.09%.
Embodiment 3
[0062] The present embodiment discloses the preparation method of the tetrandrine liposome of the present invention, specifically as follows:
[0063] Weigh soybean lecithin (0.36g), oleic acid (0.154g), cholesterol (0.036g), Tween-80 (0.072g), tetrandrine (0.06g), add absolute ethanol (30mL) to fully dissolve, The solvent was removed by evaporation under reduced pressure, and a uniform film was formed on the bottom of the bottle. Add pH 6.5 phosphate buffer (30 mL), and hydrate at 30° C. for 15 minutes to obtain liposome colostrum. Then through 500bar high-pressure homogenization three times, each homogenization time is 32s, namely the tetrandrine liposome solution. The measured particle size is 182.9nm (PDI=0.099), the potential is -16.1mV, the encapsulation efficiency is 98.62%, and the drug loading is 9.06%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com