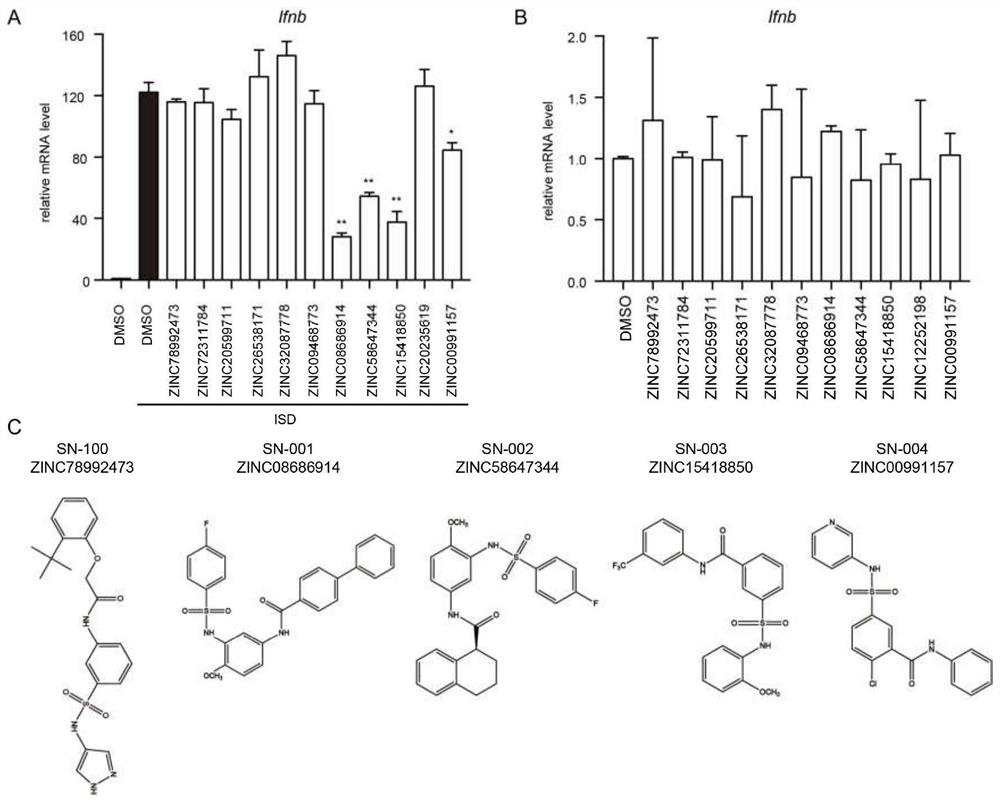
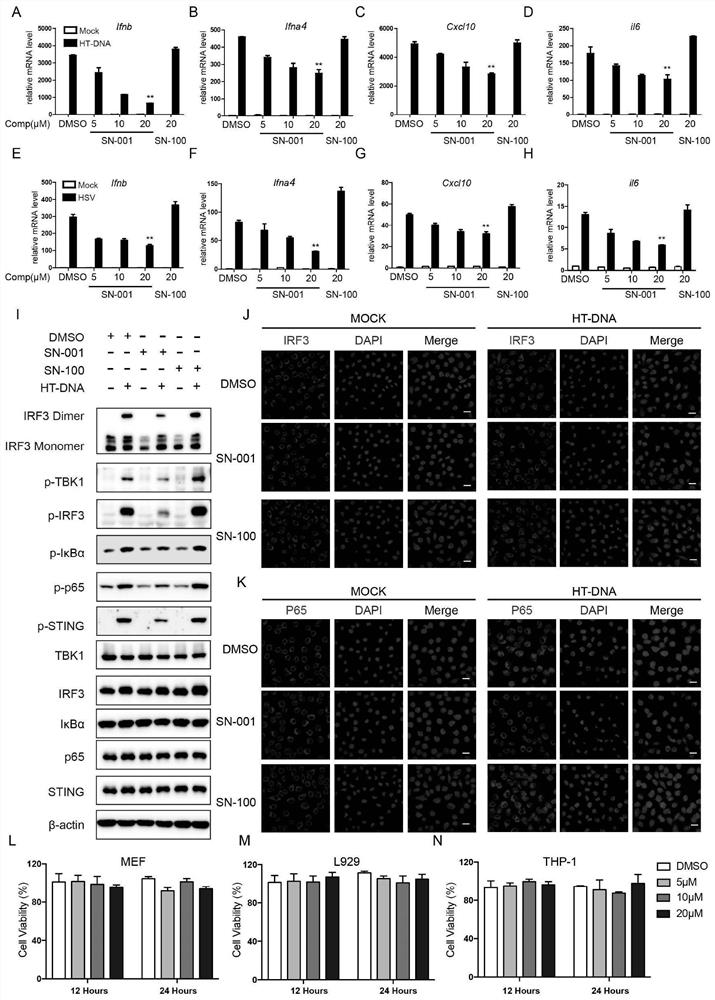
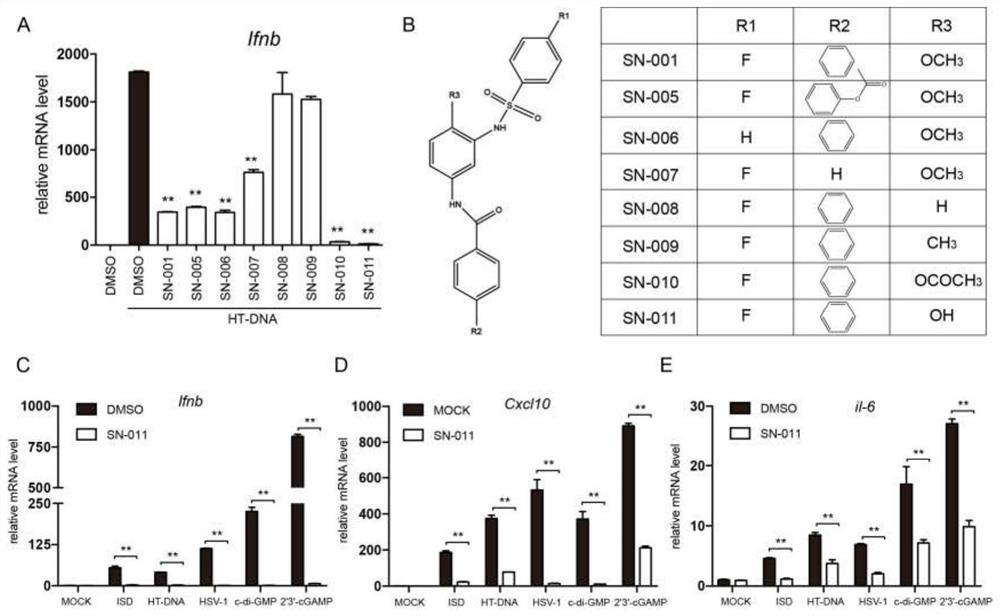
Medical application of benzenesulfonamide compound, and pharmaceutical composition
A technology of benzenesulfonamides and compounds, applied in the direction of drug combinations, amide active ingredients, antipyretics, etc., can solve the problems of large side effects and weak activity, and achieve the effect of prolonging the long-term survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] N-(3-((4-fluorophenyl)sulfonylamino)-4-hydroxyphenyl)-[1,1'-biphenyl]-4-carboxamide (Compound I)
[0127]
[0128] Compound 1 (2.00g, 13.0mmol) and pyridine (1.54g, 19.5mmol, 1.57mL) were dissolved in 40mL of dichloromethane, and 4-fluorine dissolved in 20mL of dichloromethane was added dropwise at 0°C Benzenesulfonyl chloride 1A (3.04g, 15.68mmol), mixed and stirred at 25°C for 12 hours. Subsequently, the reaction was identified to be complete by thin layer chromatography (TLC), and the mobile phase was petroleum ether:ethyl acetate=2:1. The solvent was evaporated and removed under reduced pressure, and the crude product was purified by silica gel column chromatography (mobile phase: petroleum ether: ethyl acetate = 10:1~2:1, V / V) to obtain a pale yellow solid product Compound 2 (2.74g, 67.6% yield). 1 HNMR: (DMSO-d 6 ,400MHz)δ8.04(d,J=2.4Hz,1H),7.94~7.92(m,1H),7.83~7.80(m,2H),7.41~7.37(m,2H),6.89(d,J= 8.8Hz,1H).LCMS(m / z):312.02[M+H] + .
[0129] Compound 2 (1...
Embodiment 2
[0134] N-(3-((4-fluorophenyl)sulfonylamino)-4-methoxyphenyl)-[1,1'-biphenyl]-4-carboxamide (Compound II)
[0135]
[0136] Compound 6 (0.54g, 3.2mmol), pyridine (0.5mL) was added in dichloromethane (10mL), and 4-fluorobenzenesulfonyl chloride (1A: 0.744g, 3.84mmol) was added dropwise in dichloromethane ( 10mL) solution, after dropping, react at room temperature for 8h. The solvent was evaporated under reduced pressure, ethyl acetate (50mL) and water (20mL) were added, shaken evenly to separate the liquids, the organic phase was washed with 1N HCl (10mL), water (20mL) and saturated brine (20mL) respectively, anhydrous Na 2 SO 4 Dry, filter, evaporate the solvent under reduced pressure to obtain a residue, and the crude product is purified by silica gel column chromatography (mobile phase is petroleum ether:ethyl acetate=10:1~2:1, V / V) to obtain a light yellow solid product compound 7 (0.74 g, 71%).
[0137] Compound 7 (0.5g, 1.53mmol), 10%Pd / C (50mg) was added in MeOH (20...
Embodiment 3
[0140] 4-([1,1'-biphenyl]-4-carboxamido)-2-((4-fluorophenyl)sulfonylamino)phenylacetate (Compound V)
[0141]
[0142]
[0143] Compound I (200mg, 0.43mmol) prepared in Example 1 was dissolved in THF (5mL), pyridine (52μL, 0.65mmol) was added, and acetyl chloride (37μL, 0.52mmol) was added dropwise at room temperature, and reacted at room temperature for 10h. Quenched with water (10 mL), extracted with EtOAc (30 mL), the organic phase was washed successively with 1N HCl (10 mL), water (10 mL) and saturated brine (10 mL), anhydrous Na 2 SO 4 After drying, filtering, and evaporating the solvent under reduced pressure, a white solid was obtained, which was purified by silica gel column chromatography (DCM / MeOH=10 / 1) to obtain a white solid, compound V (76 mg, 35%). 1 H NMR (300MHz, DMSO-d 6 )δppm 10.39(s,1H),10.01(s,1H),8.06(d,J=8.3Hz,2H),7.96(d,J=2.2Hz,1H),7.86-7.8(m,4H),7.77 (d,J=7.4Hz,2H),7.62(dd,J=8.8,2.3Hz,1H),7.52(dd,J=7.4,7.4Hz,2H),7.44(d,J=5.3Hz,1H) ,7.40(dd,J=7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com