Multi-shell nickel-based nitride nanocomposite material and its preparation method and application
A technology of nanocomposite materials and nitrides, which is applied in the field of multi-shell nickel-based nitride nanocomposites and its preparation, can solve the problems of limited catalytic activity and complicated preparation methods, and achieve enriched channels, improved activity and stability, The effect of large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
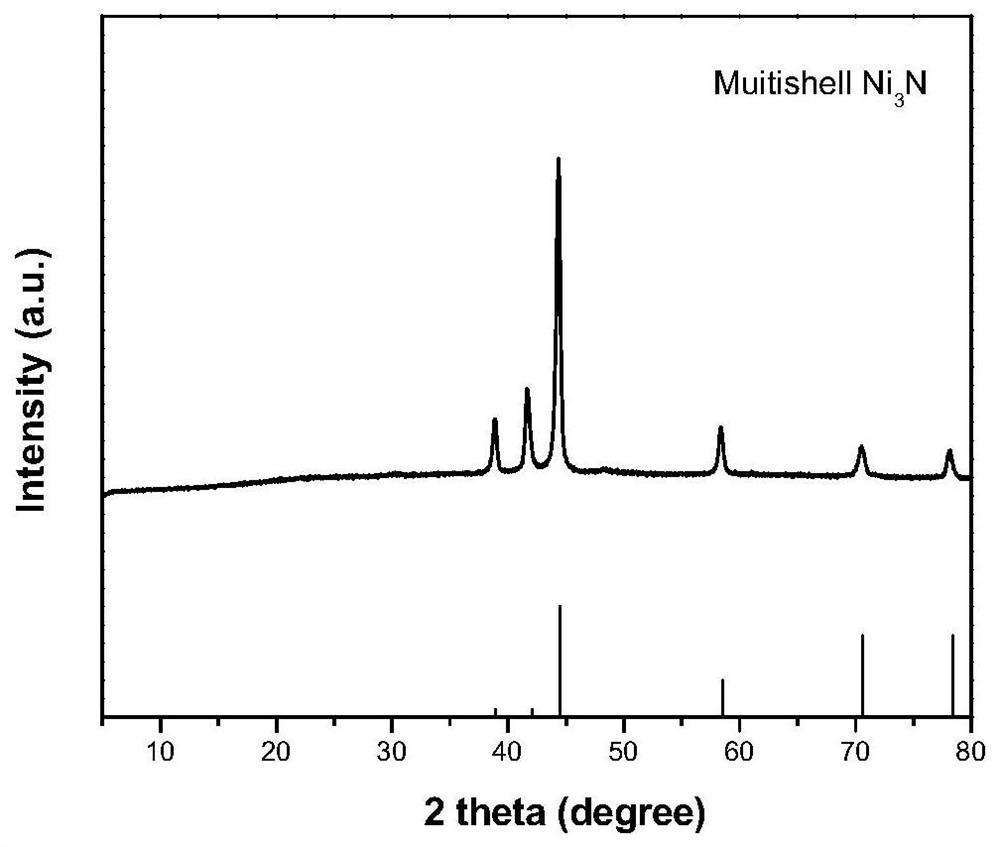
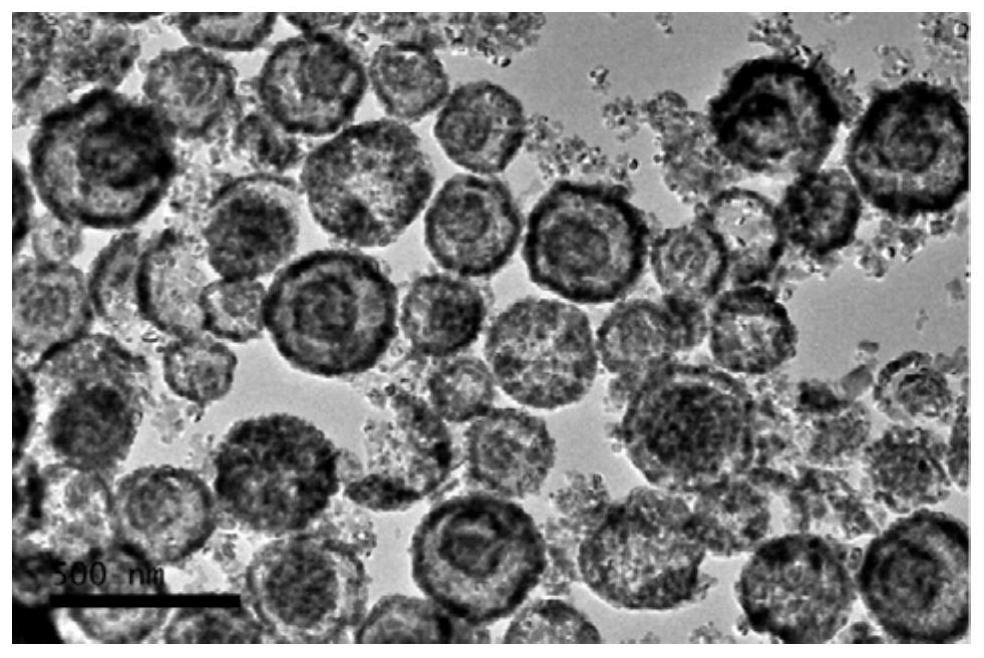
[0058] Embodiment 1 A kind of multi-shell nickel nitride nanomaterial
[0059] The preparation method of the multi-shell nickel nitride nanomaterial comprises the following steps:
[0060] S1, 1.33g (5mmol) of nickel acetate [Ni(CH 3 COO) 2 4H 2 O], 0.5g trimesic acid (H 3 BTC) and 5 g of PVP were dispersed in 500 mL of N,N-dimethylamide (DMF), ultrasonicated for 0.5 h and then vigorously stirred for 0.5 h (the stirrer speed was 900 rpm) to obtain a uniform suspension A;
[0061] S2. Transfer the suspension A obtained in step S1 to a polytetrafluoroethylene high-pressure reactor, seal it, and place it in a blast drying oven for solvothermal reaction. React at 150°C for 6 hours. After the reaction is completed, let it cool naturally. The final material was suction-filtered through an organic hybrid nylon new sub-filter membrane with a pore size of 0.45 μm, washed with ethanol for 3 times, and dried in an oven at 80°C for 12 hours to obtain a transition metal nickel ion coor...
Embodiment 2
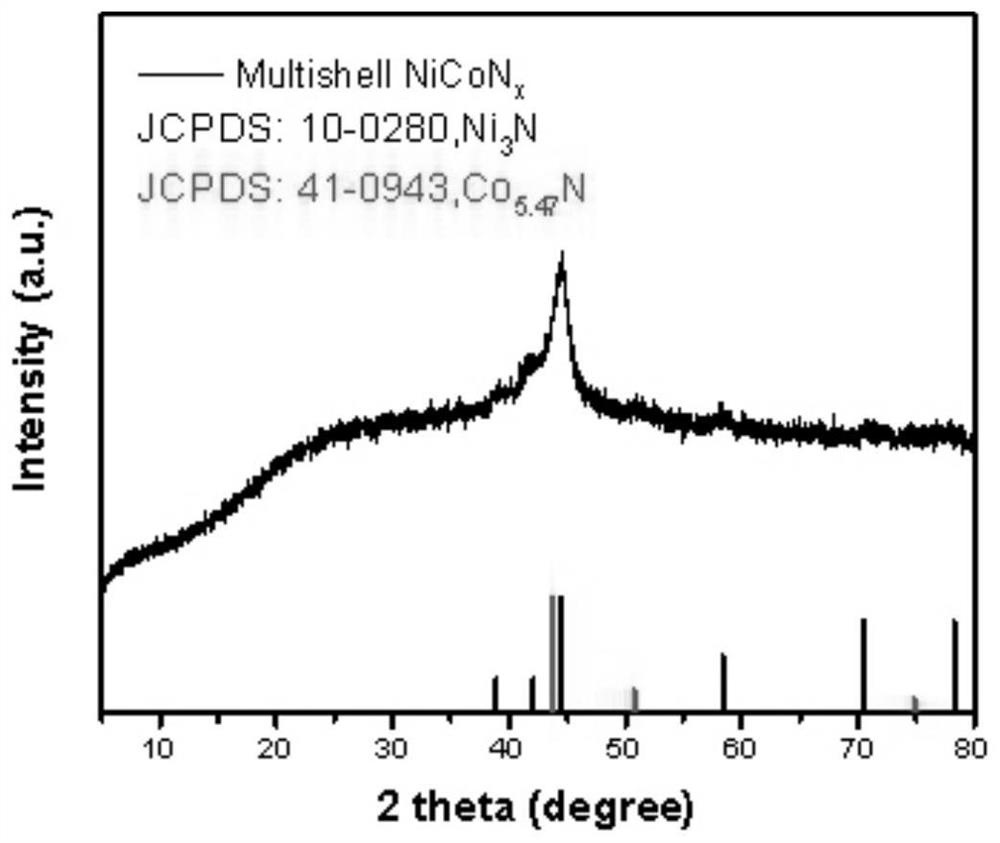
[0067] Embodiment 2 A kind of multi-shell nickel-cobalt double metal nitride hollow structure nanomaterial
[0068] The preparation method of the multi-shell nickel-cobalt double metal nitride hollow structure nanomaterial comprises the following steps:
[0069] S1, 2.37g (10mmol) of nickel chloride [NiCl 2 ·6H 2 O], 1.18g (5mmol) cobalt chloride [CoCl 2 ·6H 2 O], 1g of tannic acid (TA) and 2g of SDS were dispersed in 500mL of deionized water, and then it was ultrasonically stirred for 0.1h and then vigorously stirred for 1h (the stirring speed was 900rpm) to obtain a uniform suspension A;
[0070] S2. Transfer the suspension A obtained in step S1 to a polytetrafluoroethylene high-pressure reactor, seal it and place it in a blast drying oven for solvothermal reaction, and react at 100°C for 12 hours. After the reaction is completed, let it cool naturally. The final materials were washed three times with deionized water, centrifuged at 10,000 rpm each time, and dried in an ...
Embodiment 3
[0075] Example 3 A multi-shell nickel-copper bimetallic nitride hollow structure nanomaterial
[0076] The preparation method of the multi-shell nickel-copper double metal nitride hollow structure nanomaterial comprises the following steps:
[0077] S1, 1.16g (4mmol) nickel nitrate [Ni(NO 3 ) 2 ·6H 2 O], 1.13g (6mmol) copper nitrate [Cu(NO 3 ) 2 ·6H 2 O], 4g Schiff's base (SB) and 4g CTAB were dispersed in 80mL acetone, and then it was ultrasonically stirred for 1h and then vigorously stirred for 1.5h (stirrer speed was 900rpm) to obtain a uniform suspension A;
[0078]S2. Transfer the suspension A obtained in step S1 to a polytetrafluoroethylene high-pressure reactor, seal it, and place it in a blast drying oven for solvothermal reaction. React at 150°C for 6 hours. After the reaction is completed, let it cool naturally. The final material was suction-filtered through an organic hybrid nylon new sub-filter membrane with a pore size of 0.45 μm, washed with ethanol for 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pore size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com