Recombinant expression vector, chimeric antigen receptor T cell with reduced depletion and applications of T cell
A chimeric antigen receptor and expression vector technology, applied in genetically modified cells, cells modified by introducing foreign genetic material, vectors, etc., can solve T cell exhaustion, T cell exhaustion that has not been disclosed, CAR-T cells Loss of effect function, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
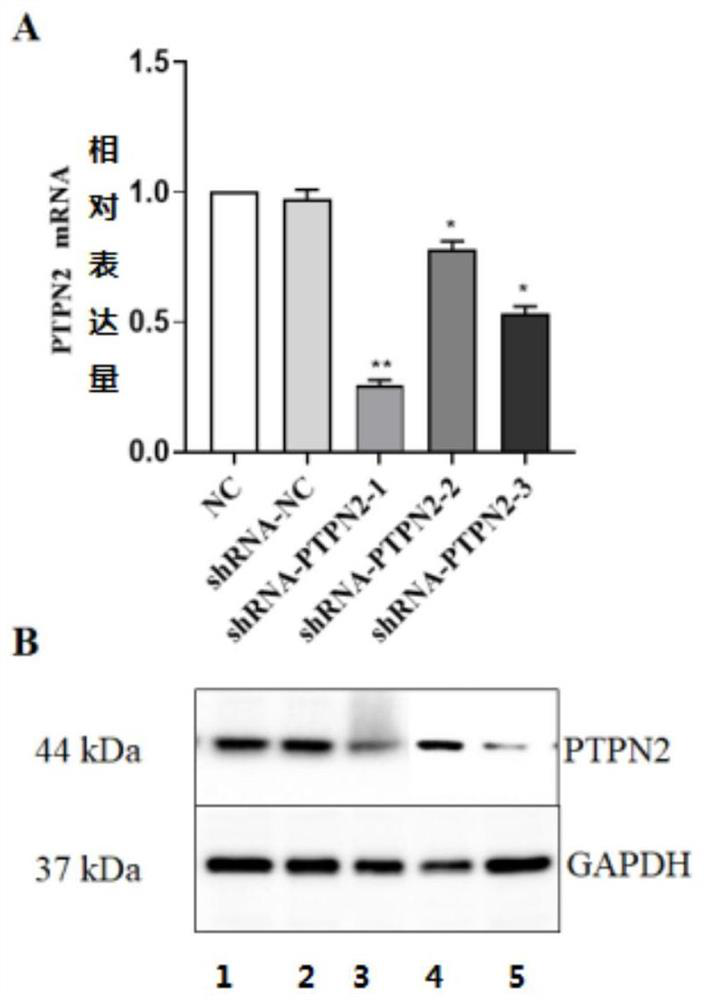
[0053] Determine the effective target sequence of the PTPN2 gene, add restriction endonuclease Eco I and BspEI sites at its 5' end and 3' end, synthesize the required DNA double-stranded fragment, and obtain 3 candidate sequences, named sh -PTPN2-1, sh-PTPN2-2, sh-PTPN2-3, the complementary DNA sequences of each group are as follows:
[0054] sh-PTPN2-1:
[0055] 5'-CCGGC GCTATTACTACCTTTAATTCATTTTTTG-3';
[0056] 5'-AATTCAAAAAA AATTAAAGGTAGTAATAGCCC-3';
[0057] sh-PTPN2-2:
[0058] 5'-CCGGC CGTATCAACAATGTTCGATGTTTTTTTTG-3';
[0059] 5'-AATTCAAAAAAATCGAACATTGTTGATACGAA-3';
[0060] sh-PTPN2-3:
[0061] 5'-CCGGC CCAGGATCAATCATGTCATTCTTTTTTG-3';
[0062] 5'-AATTCAAAAAA ATGACATGATTGATCCTGGAT-3'.
[0063] Culture 293T cells in logarithmic growth phase, inoculate 1.3-1.5×10 cells 2 days before transfection 6 Put each cell into a 10cm culture dish, add 10mL DMEM medium containing 10% FBS (fetal bovine serum is heat-inactivated in advance), and wait until the cells grow to 70...
Embodiment 2
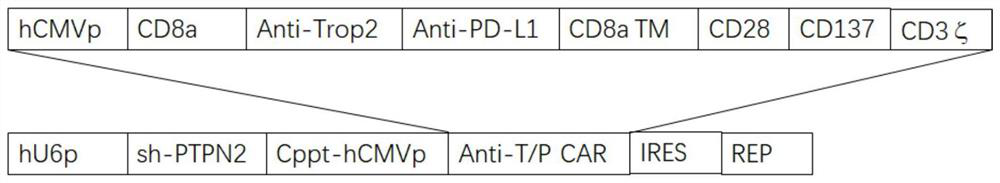
[0075] 1. Western-blot detection of the expression of CD3ζ and PTPN2 in CAR-T cells
[0076] Extract bispecific chimeric antigen receptor retroviral plasmid, transfect into human embryonic kidney cell 293T cells with PI transfection reagent, wash once with PBS after 48 hours, lyse cells with cell protein extraction reagent RIPA, extract transfection The proteins of the 293T cells were subjected to gel electrophoresis by 10% SDS-PAGE, transferred to the membrane, incubated with mouse anti-human CD3ζ antibody and PTPN2 antibody at 4°C overnight, and then used horseradish peroxidase-labeled anti-mouse II Anti-incubation for 1h, ECL color development.
[0077] The result is as Figure 5 As shown, the anti-human CD3ζ antibody can detect the expression of CAR molecules, the protein molecular size is consistent with the theory, which is 75kDa, and the position of PTPN2 is about 46kDa.
[0078] 2. Flow cytometric detection of the expression of PTPN2 expression molecules in CAR-T cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com