Taxane drug and doxorubicin prodrug self-assembled nanoparticles and application thereof
A technology for self-assembling nanoparticles and taxanes, which is used in the preparation of taxane drugs-adriamycin prodrug self-assembled nanoparticles, application in drug delivery systems, taxane drugs-doxorubicin In the field of self-assembled nanoparticles of prodrugs, it can solve the problems of suboptimal efficacy, inconsistent release rate, and sudden release, and reduce the effect of intravenous injection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
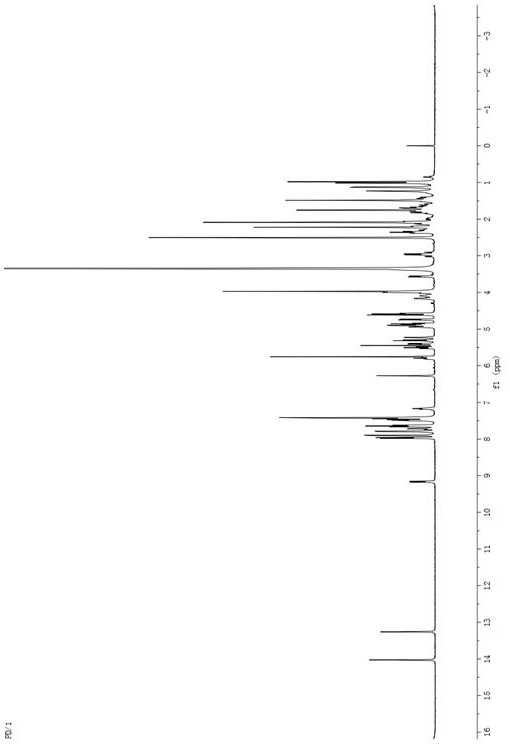
[0068] Embodiment 1: the synthesis of paclitaxel-doxorubicin prodrug PTX-C-DOX
[0069] A certain amount of paclitaxel and glutaric anhydride were dissolved in a small amount of dichloromethane, and under the catalysis of 4-dimethylaminopyridine (DMAP), the reaction was stirred at room temperature under nitrogen protection for 24 hours. After the reaction was completed, the reaction solution was washed three times with saturated brine. Separation of CH 2 Cl 2 layer and dried over anhydrous sodium sulfate, filtered, concentrated and evaporated to dryness with a rotary evaporator, separated and purified to obtain intermediate 1 as a white solid. Appropriate amount of intermediate 1 and doxorubicin were mixed in dichloromethane, added HBTU and DIPEA and stirred together, and stirred under nitrogen for 1 day. After the reaction, the dichloromethane layer was washed three times with saturated brine, dried over anhydrous sodium sulfate, filtered, evaporated to dryness, and separat...
Embodiment 2
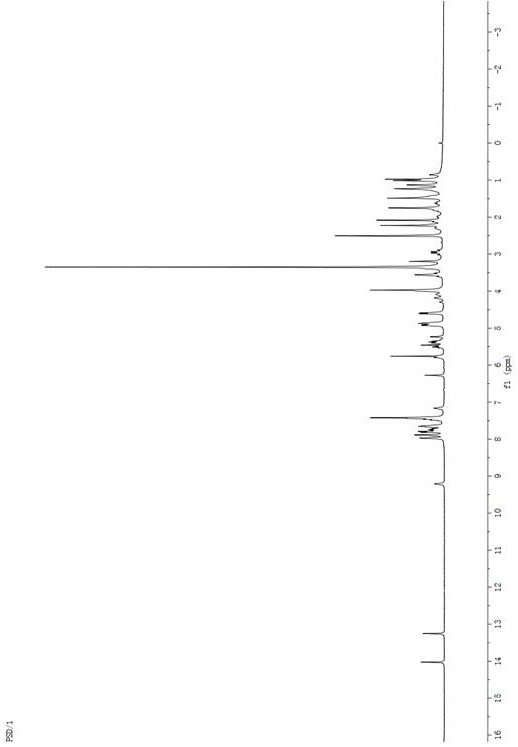
[0071] Example 2: Synthesis of Paclitaxel-Adriamycin prodrug PTX-S-DOX linked by redox double sensitive thioether bond
[0072] Dissolve an appropriate amount of paclitaxel and thioglycolic anhydride in a small amount of dichloromethane, under the catalysis of 4-dimethylaminopyridine (DMAP), stir and react at room temperature under nitrogen protection for 24 hours, after the reaction is completed, wash the reaction solution with saturated brine three times , the CH2Cl2 layer was separated and dried with anhydrous sodium sulfate, filtered, concentrated and evaporated to dryness with a rotary evaporator, and separated and purified to obtain intermediate 2 as a white solid. Appropriate amount of intermediate 2 and doxorubicin were mixed in dichloromethane, added HBTU and DIPEA and stirred together, and stirred under nitrogen for 1 day. After the reaction, the dichloromethane layer was washed three times with saturated brine, dried over anhydrous sodium sulfate, filtered, evaporat...
Embodiment 3
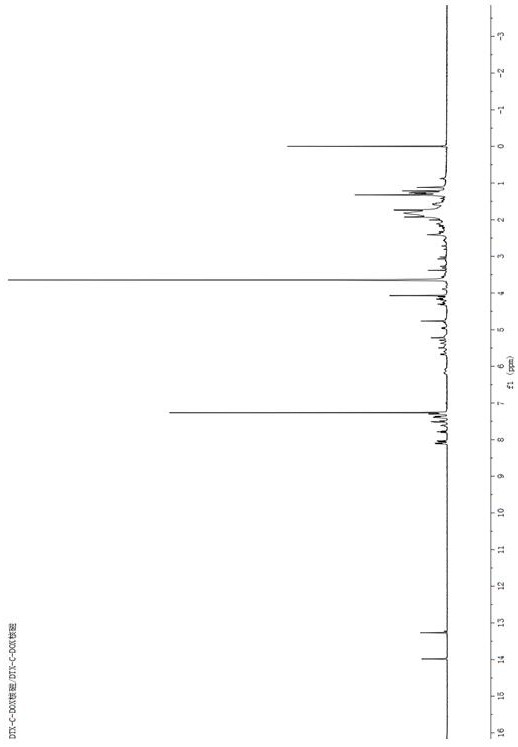
[0074] Embodiment 3: the synthesis of docetaxel-doxorubicin prodrug DTX-C-DOX
[0075]Dissolve a certain amount of docetaxel and glutaric anhydride in a small amount of dichloromethane, and under the catalysis of 4-dimethylaminopyridine (DMAP), stir and react at room temperature under nitrogen protection for 24 hours. After the reaction, the reaction solution is washed with saturated brine After washing three times, the CH2Cl2 layer was separated and dried with anhydrous sodium sulfate, filtered, concentrated and evaporated to dryness with a rotary evaporator, separated and purified to obtain intermediate 3 as a white solid. An appropriate amount of intermediate 3 and doxorubicin were mixed in dichloromethane, added with HBTU and DIPEA and stirred together, and stirred under nitrogen for 1 day. After the reaction, the dichloromethane layer was washed three times with saturated brine, dried over anhydrous sodium sulfate, filtered, evaporated to dryness, and separated and purifi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com