Application of A-ring modified cryptoxine derivative in prevention and treatment of agricultural plant diseases
A technology of selemenine and derivatives, applied in the field of natural medicinal chemistry, can solve the problems of enhancing and threatening human health and environmental safety, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
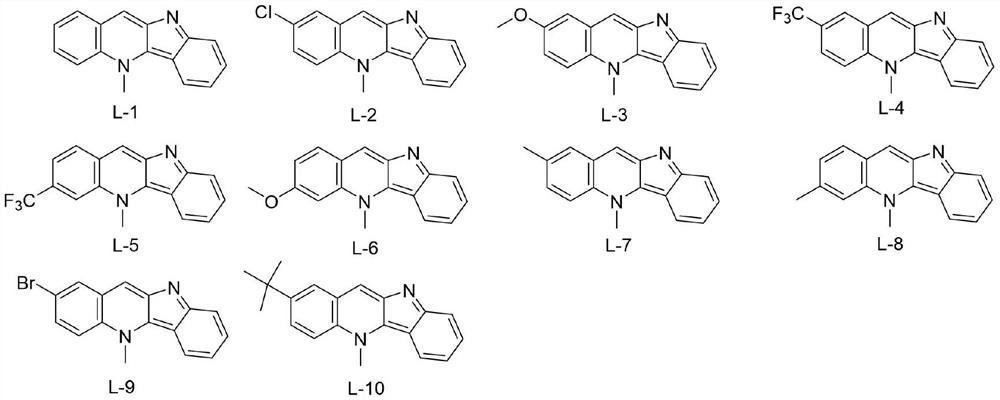
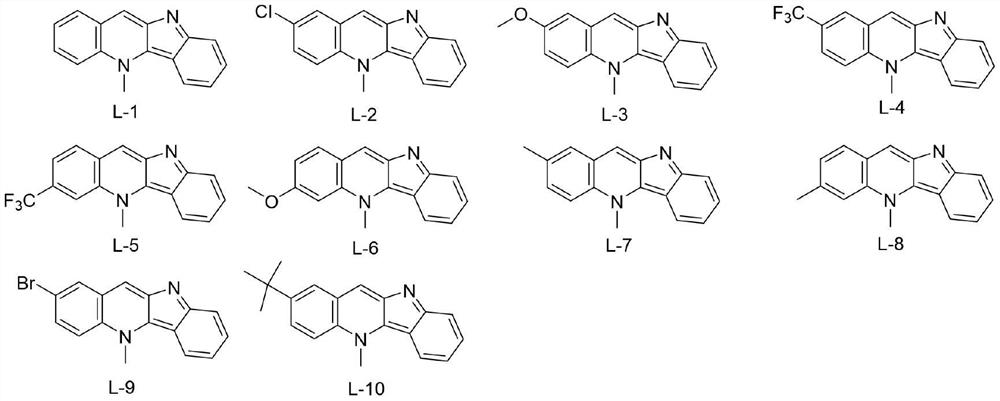
[0013] Embodiment 1: the synthesis of target compound L-1
[0014]
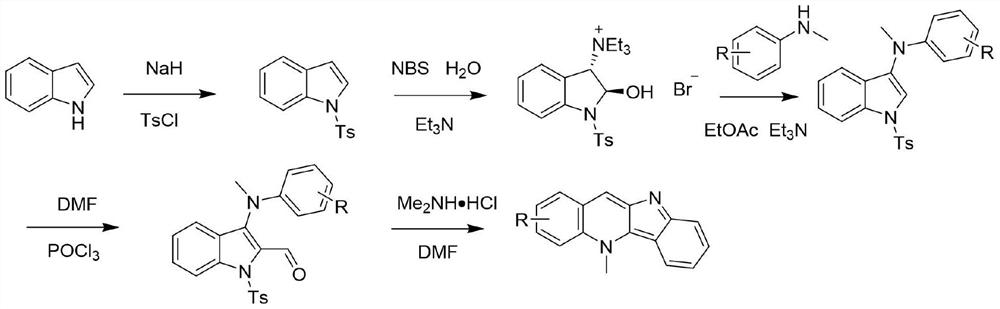
[0015] For the synthesis method, please refer to the literature method: Org.Lett.2017,19(16):4275-4278. The specific synthesis operation is as follows: Synthesis of intermediate 1: dissolve indole (30mmol) in acetonitrile, keep the system at 0°C and add 60 % sodium hydride (42mmol), add p-toluenesulfonyl chloride (33mmol) after stirring for 10 minutes, stir at room temperature for 4 hours, add saturated ammonium chloride aqueous solution to stop the reaction, then extract with ethyl acetate, wash with brine, combine the organic phases, and After drying over magnesium sulfate, it was filtered, and the organic phase was concentrated under reduced pressure to obtain a solid residue, which was purified by column chromatography using petroleum ether / ethyl acetate as eluent to obtain a white solid product.
[0016] Synthesis of Intermediate 2: Add Intermediate 1 (11mmol) and distilled water (110mmol) to 110mL of...
Embodiment 2
[0021] Embodiment 2: the synthesis of target compound L-2
[0022]
[0023] The experimental procedure is the same as in Example 1, only 4-chloro-N-methylaniline is used instead of N-methylaniline. Yield: 37%; purple-red powdery solid; 1 H NMR (400MHz, DMSO-d 6 )δ:8.88(s,1H),8.55(d,J=9.6Hz,1H),8.51(d,J=2.4Hz,1H),8.47(d,J=8.5Hz,1H),7.86(dd, J=9.4,2.5Hz,1H),7.66(d,J=8.5Hz,1H),7.55(t,J=7.6Hz,1H),7.05(t,J=7.5Hz,1H),4.90(s, 3H). 13 C NMR (100MHz, DMSO-d 6 )δ: 160.94, 145.29, 139.88, 131.46, 131.30, 128.85, 128.49, 127.98, 125.66, 125.60, 125.19, 119.59, 119.37, 117.29, 114.15, 39.88. MS-ESI m / z: C 16 h 11 ClN 2 :267.0[M+H] + .
Embodiment 3
[0024] Embodiment 3: the synthesis of target compound L-3
[0025]
[0026] The experimental procedure is the same as in Example 1, except that 4-methoxy-N-methylaniline is used instead of N-methylaniline. Yield: 38%; red-black powdery solid; 1 H NMR (400MHz, DMSO-d 6 )δ:8.81(d,J=4.5Hz,1H),8.50–8.46(m,1H),8.45(d,J=5.5Hz,1H),7.85–7.75(m,1H),7.64(d,J =8.6Hz,1H),7.53(dd,J=8.0,5.7,4.0Hz,2H),7.04(t,J=7.5Hz,1H),4.91(s,3H),3.95(s,3H). 13 C NMR (100MHz, DMSO-d 6)δ: 155.69, 145.29, 138.48, 130.41, 128.90, 126.33, 125.25, 124.93, 121.53, 119.17, 118.58, 116.59, 114.31, 107.25, 56.09, 39.48. MS-ESI m / z: C 17 h 14 N 2 O:263.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com