Disulfiram-based glucan nanometer prodrug, and preparation method and application thereof
A nano-drug, dextran technology, applied in the field of polymers, can solve the problems of limiting the clinical application of disulfiram, fast metabolism, poor stability, etc., and achieve the effects of low cost, good hydrophilicity, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
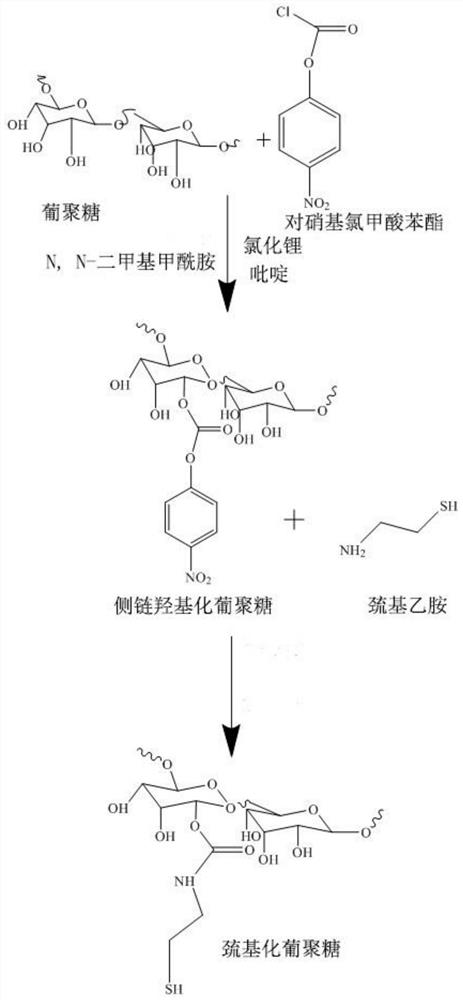
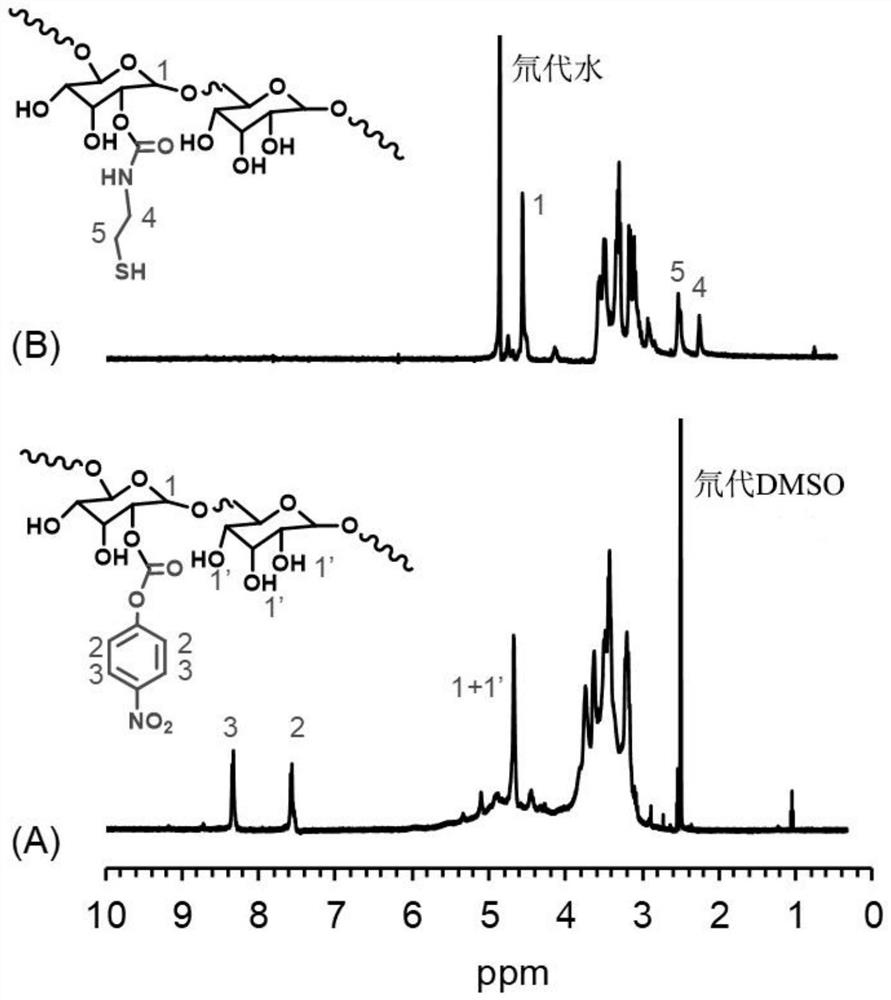
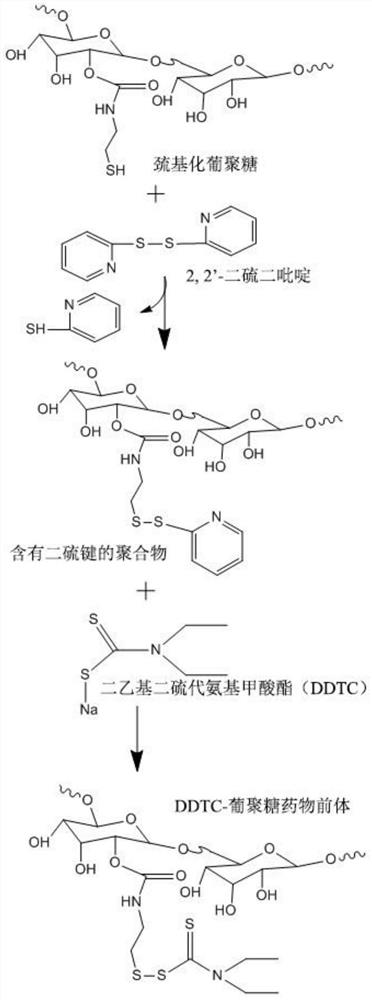
[0034] In this example, see figure 1 and image 3 , a method for preparing disulfiram-based dextran nano drug prodrugs, the steps are as follows:
[0035] (1) Preparation of side chain hydroxylated dextran:
[0036] Weigh 1g of dextran and 200mg of LiCl into a 100mL three-necked flask and place it in a vacuum drying oven, set the temperature at 75°C, and dry under vacuum for 72h; after the drying, add 10mL of anhydrous N , in N-dimethylformamide (DMF) and set the temperature of the oil bath to 90°C and stir until the liquid is clear; remove the oil bath after the liquid is clear, place it in an ice bath after standing to cool, and slowly and continuously pass nitrogen protection, Then add 0.59 mL of pyridine, so that the amount of pyridine is twice the molar amount of phenyl p-nitrochloroformate, then add 0.75 mg of phenyl p-nitrochloroformate in batches, and react under ice bath conditions (0°C) for 24 hours; The amount of phenyl p-nitrochloroformate is calculated based on...
Embodiment 2
[0053] This embodiment is basically the same as Embodiment 1, especially in that:
[0054] In this example, see figure 1 and image 3 , a method for preparing disulfiram-based dextran nano drug prodrugs, the steps are as follows:
[0055] (1) Preparation of side chain hydroxylated dextran:
[0056] Weigh 5g of dextran and 200mg of LiCl into a 100mL three-necked flask and place it in a vacuum drying oven, set the temperature at 75°C, and dry it under vacuum for 72h; after the drying, add 10mL of anhydrous N , in N-dimethylformamide (DMF) and set the temperature of the oil bath to 90°C and stir until the liquid is clear; remove the oil bath after the liquid is clear, place it in an ice bath after standing to cool, and slowly and continuously pass nitrogen protection, Subsequently, 1.18 mL of pyridine was added, so that the amount of pyridine was twice the molar amount of phenyl p-nitrochloroformate, and then 1.5 mg of phenyl p-nitrochloroformate was added in batches, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com