Catalyst for catalytic transfer hydrogenation and preparation method and application thereof
A transfer hydrogenation and catalyst technology, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, catalytic reactions, etc., can solve the problems of by-products, high temperature and pressure, and catalyst reactions Low activity and other problems, to achieve the effect of high catalytic transfer hydrogenation activity, high selectivity, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
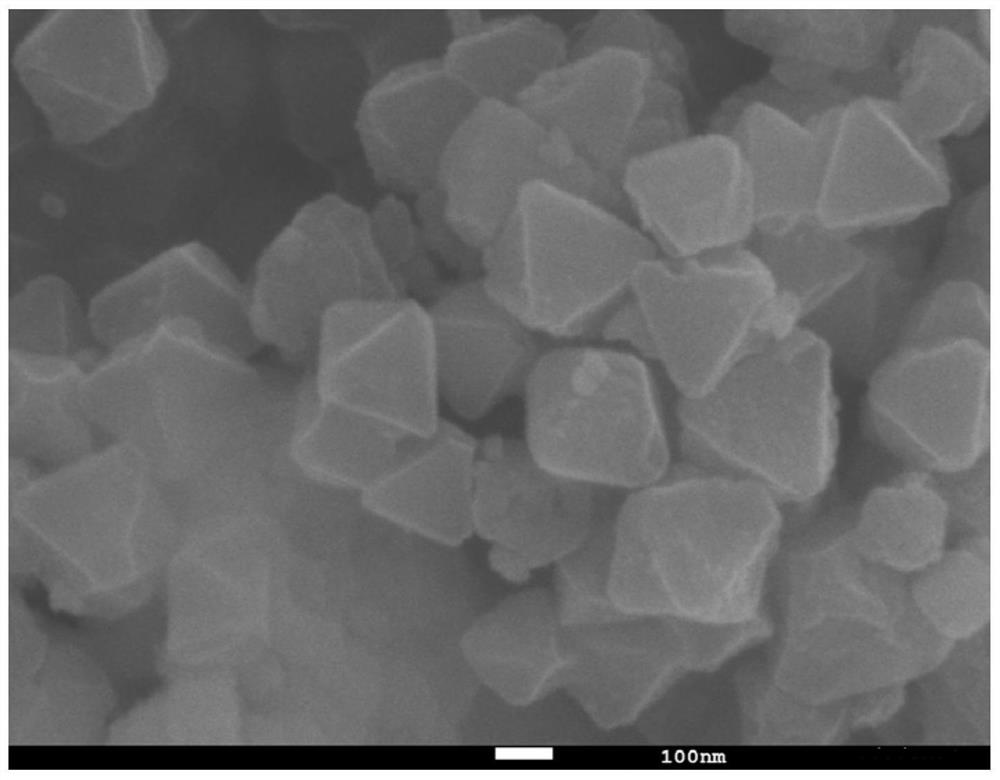
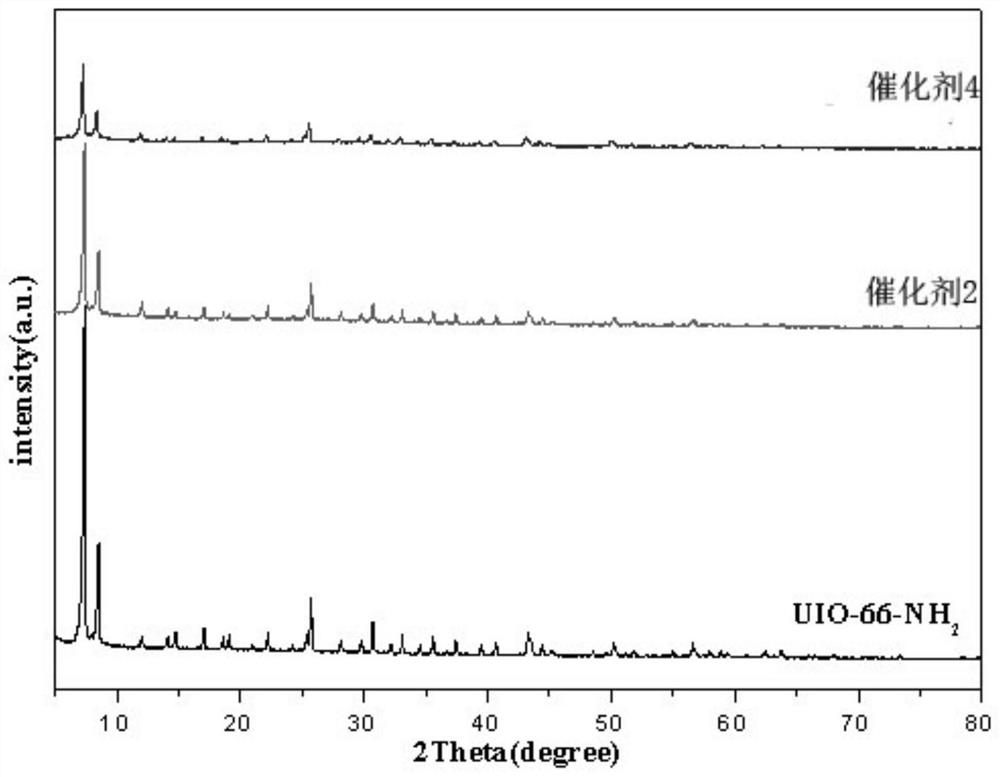
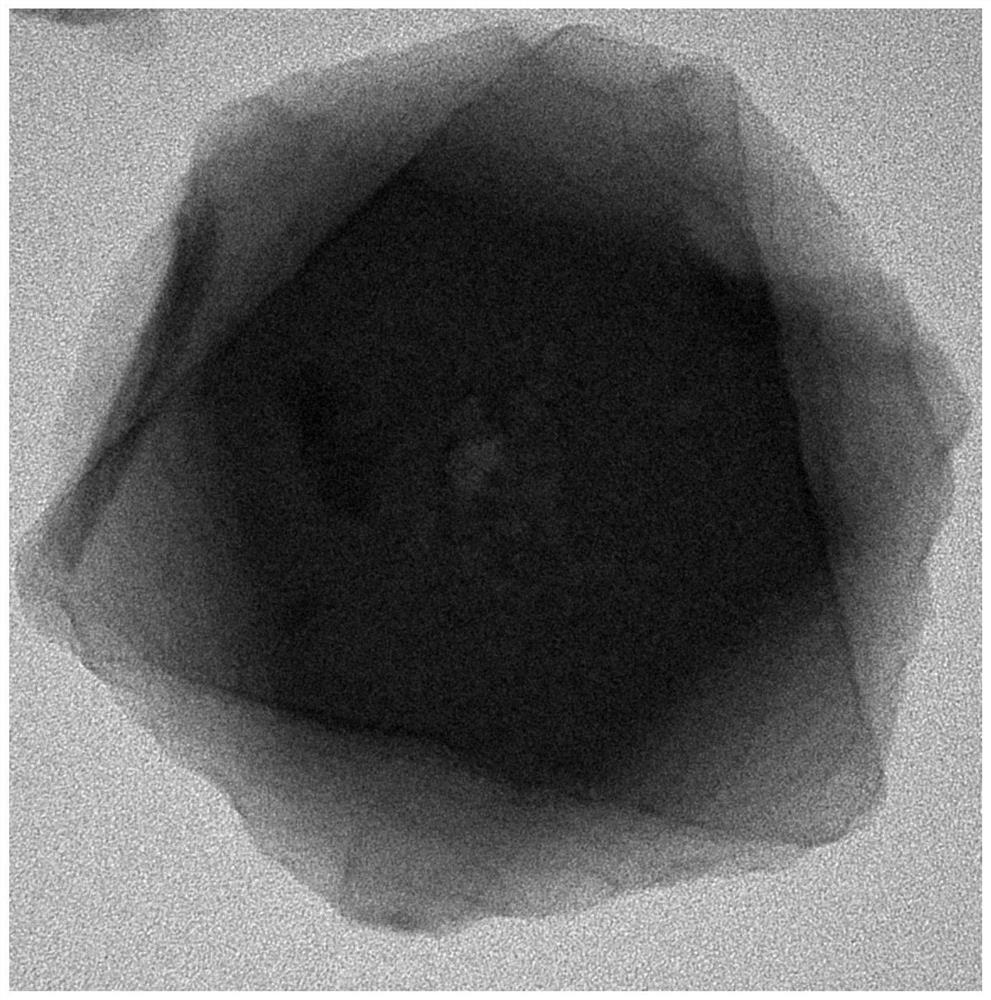
[0039] (1) Dissolve 1.119g of zirconium chloride and 0.87g of 2-aminoterephthalic acid in 60ml of N,N-dimethylformamide respectively, and stir at 400rpm for 30 minutes to obtain zirconium chloride solution and 2-aminoterephthalic acid Terephthalic acid solution, then 2-aminoterephthalic acid solution was added dropwise to the zirconium chloride solution at a speed of 5mL / min, after mixing and stirring evenly, 28ml of glacial acetic acid solution was added thereto, and after stirring for 30 minutes, Ultrasonic treatment at 200W for another 20 minutes to obtain a mixed solution; then transfer the mixed solution to a stainless steel sealed polytetrafluoroethylene reactor and react at 120°C for 24 hours. After the reactor is cooled, the reaction solution is centrifuged. The obtained solid was washed three times with N,N-dimethylformamide and methanol, and the precipitate was dried in vacuum at 60°C for 24 hours to obtain the precursor UIO-66-NH 2 .
[0040](2) Take 0.247g cobalt ...
Embodiment 2
[0042] (1) Dissolve 1.119g of zirconium chloride and 1.305g of 2-aminoterephthalic acid in 60ml of N,N-dimethylformamide respectively, stir at 300rpm for 40 minutes to obtain zirconium chloride solution and 2-aminoterephthalic acid Terephthalic acid solution, and then 2-aminoterephthalic acid solution was added dropwise to the zirconium chloride solution at a speed of 4mL / min. After mixing and stirring evenly, 28ml of glacial acetic acid solution was added thereto, and after stirring for 45 minutes, the The power is 300W and then ultrasonic treatment for 15min to obtain the mixed solution, and then the mixed solution is transferred to a stainless steel sealed polytetrafluoroethylene reactor, and reacted at 120°C for 48 hours, after the reactor is cooled, the reaction solution is centrifuged to obtain The solid was washed three times with N,N-dimethylformamide and ethanol, and the precipitate was vacuum-dried at 80°C for 12 hours to obtain the precursor UIO-66-NH 2 .
[0043] ...
Embodiment 3
[0045] (1) Dissolve 1.119g of zirconium chloride and 0.87g of 2-aminoterephthalic acid in 60ml of N,N-dimethylformamide respectively, and stir at 400rpm for 30 minutes to obtain zirconium chloride solution and 2-aminoterephthalic acid Terephthalic acid solution, and then 2-aminoterephthalic acid solution was added dropwise to the zirconium chloride solution at a speed of 5mL / min. After mixing and stirring evenly, 28ml of glacial acetic acid solution was added thereto, and after stirring for 30 minutes, the The power is 400W and then ultrasonic treatment for 10min to obtain the mixed solution, then the mixed solution is transferred to a stainless steel sealed polytetrafluoroethylene reactor, and reacted at 120°C for 24 hours, after the reactor is cooled, the reaction solution is centrifuged to obtain The solid was washed three times with N,N-dimethylformamide and methanol, and the precipitate was vacuum-dried at 60°C for 24 hours to obtain the precursor UIO-66-NH 2 .
[0046] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com